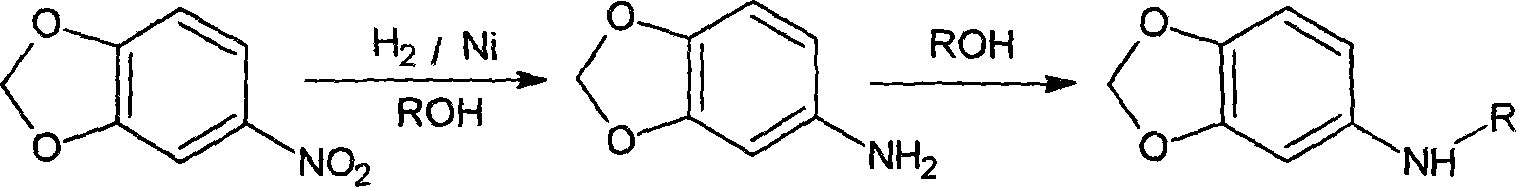
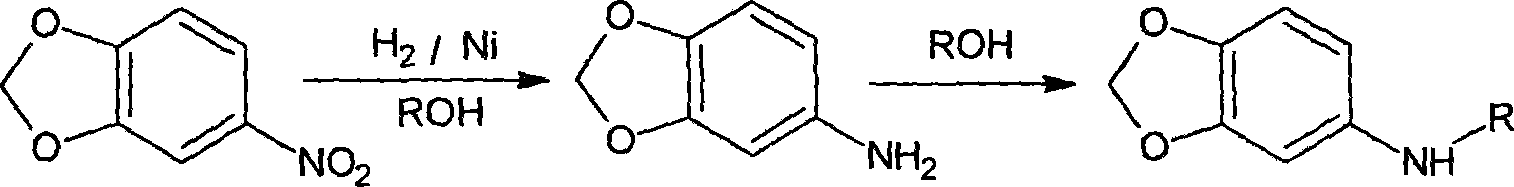
Method for producing N-alkyl-(3,4-methylenedioxy) aniline
A methylene dioxy, alkyl technology, applied in the field of N-alkyl-aniline, can solve problems such as complicated operation, achieve the effects of good selectivity, less environmental pollution, and lower production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment
[0013] Example 1. Take 125g of 3,4-(methylenedioxy)nitrobenzene, 25g of Raney nickel catalyst, and 275g of dehydrated ethanol and put them into an autoclave, feed hydrogen, and react at 80°C / 2.5MPa for 2 hours. Replaced with nitrogen, raised the temperature to 170°C, and reacted for 10 hours. The product was concentrated and distilled under high vacuum, and 115g of fractions between 130-150°C were collected, analyzed by gas chromatography, the content of N-ethyl-(3,4-methylenedioxy)aniline was 96.4%, and the yield was 93.1% . 3,4-methylenedioxyaniline was not detected.
[0014] Example 2, 125g of 3,4-(methylenedioxy)nitrobenzene, 25g of Raney catalyst, and 275g of methanol were mixed and put into an autoclave, and hydrogen was passed through to react at 70°C / 2.5MPa for 2.5 hours. Replaced with nitrogen, raised the temperature to 160°C, and reacted for 10 hours. The product was concentrated and distilled under high vacuum, and 106g of fractions between 125-145°C were collecte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com