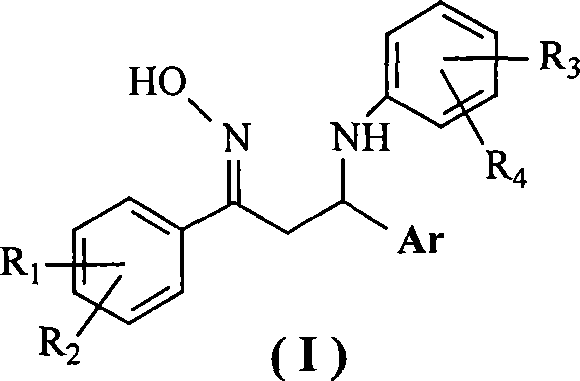
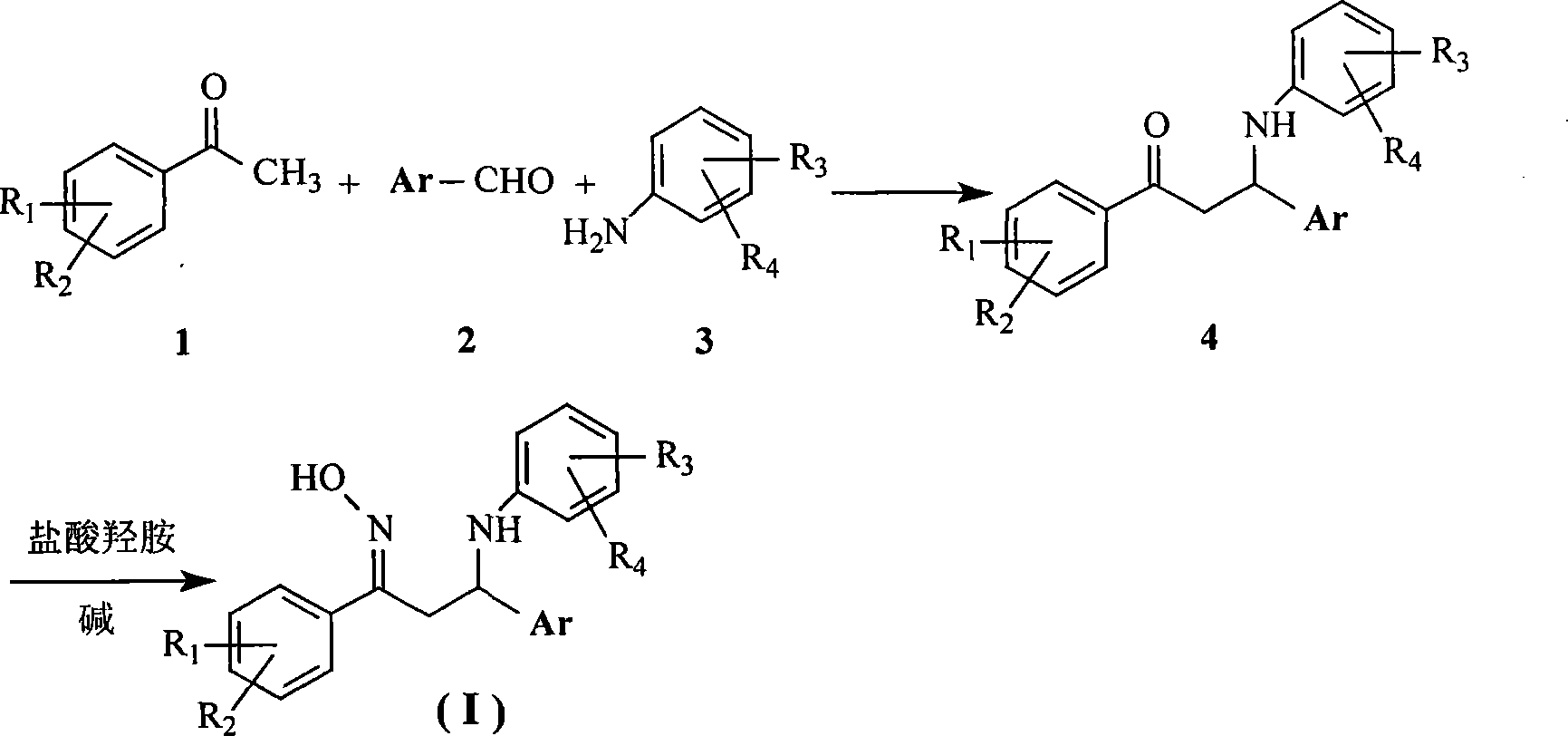
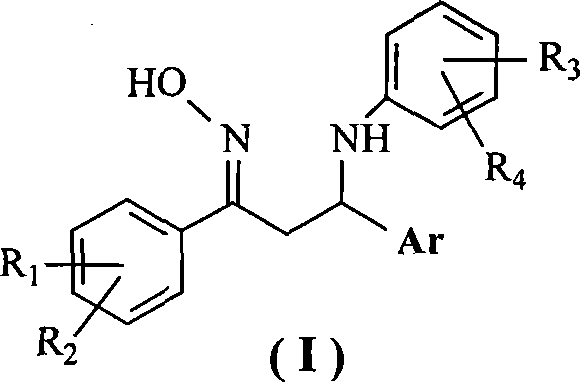
1,3-diaryl-3-aryl amidine-1-acetoxime compounds, preparation method and use thereof
An acetone oxime and compound technology, applied in the field of medicinal chemistry, can solve the problems of gastrointestinal discomfort, tumor volume increase, rise and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Preparation of 1-(4-nitrophenyl)-3-phenyl-3-(4-chloroanilino)-1-acetone oxime
[0028]
[0029] Add 1.06 g (0.01 mol) of benzaldehyde, 1.28 g (0.01 mol) of 4-chloroaniline and 18 ml of absolute ethanol in the reaction flask, stir at room temperature for 10 minutes, then add 1.65 g (0.01 mol) of 4-nitroacetophenone And catalytic amount of concentrated hydrochloric acid, stirred at room temperature; the precipitated solid was filtered by suction and washed with absolute ethanol. The obtained solid was suspended in 20ml ethanol and washed with saturated NaHCO 3 The aqueous solution was neutralized to alkaline, filtered with suction, and the filter cake was washed with a small amount of absolute ethanol. The crude product was recrystallized in a mixed solvent of ethanol / water (volume ratio 1:1) to obtain 3.43 g of a light yellow powder solid, with a yield of 83%.
[0030] 1.9 grams (5 mmol) of the above-mentioned solid and 20 ml of ethanol were added to the re...
Embodiment 2
[0031] Example 2 Preparation of 1-(4-methylphenyl)-3-(2-thienyl)-3-(4-chloroanilino)-1-acetone oxime
[0032]
[0033] The operation process is the same as in Example 1, except that benzaldehyde is replaced by 2-thiophene formaldehyde, and 4-nitroacetophenone is replaced by 4-methylacetophenone to obtain white crystals with a yield of 85.6%; mp117~119°C; 1 H-NMR (400MHz, CDCl 3 )δ: 7.44(d, 2H, J=8.4Hz), 7.19(d, 2H, J=8Hz), 7.16(d, 1H, J=4.4Hz), 6.99(m, 3H), 6.92(t, 1H , J=3.6Hz), 6.39(d, 2H, J=8.4Hz), 4.89(dd, 1H, J=4.4Hz), 3.69(dd, 1H, J 1 =13.6Hz,J 2 =10Hz), 3.11(dd,1H,J 1 =13.6Hz,J 2 =4.4Hz), 2.38(s, 3H); ESI-MS m / z: 404.2(M + +H+MeOH).
Embodiment 3
[0034] Example 3 Preparation of 1-(4-methylphenyl)-3-phenyl-3-(4-chloroanilino)-1-acetone oxime
[0035]
[0036] The operation process is the same as in Example 1, except that 4-nitroacetophenone is replaced by 4-methylacetophenone to obtain light yellow crystals with a yield of 93.4%; mp156~158°C; 1 H-NMR (400MHz, CDCl 3 )δ: 7.48(d, 2H, J=8.4Hz), 7.38(d, 2H, J=6.8Hz), 7.32(t, 2H, J=7.2Hz), 7.26~7.20(m, 3H), 6.93( d, 2H, J=8.8Hz), 6.28(d, 2H, J=9.2Hz), 4.55(dd, 1H, J 1 =10.4Hz,J 2 =4Hz), 3.59(dd,1H,J 1 =13.6Hz,J 2 = 10.4Hz), 2.97(dd, 1H, J 1 =13.8Hz,J 2 =4Hz), 2.38(s, 3H); ESI-MS m / z: 365.9(M + +H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com