Recombination bacillary viral vector skeleton plasmid and application thereof
A technology for recombining baculovirus and backbone plasmid, applied in the field of animal genetic engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
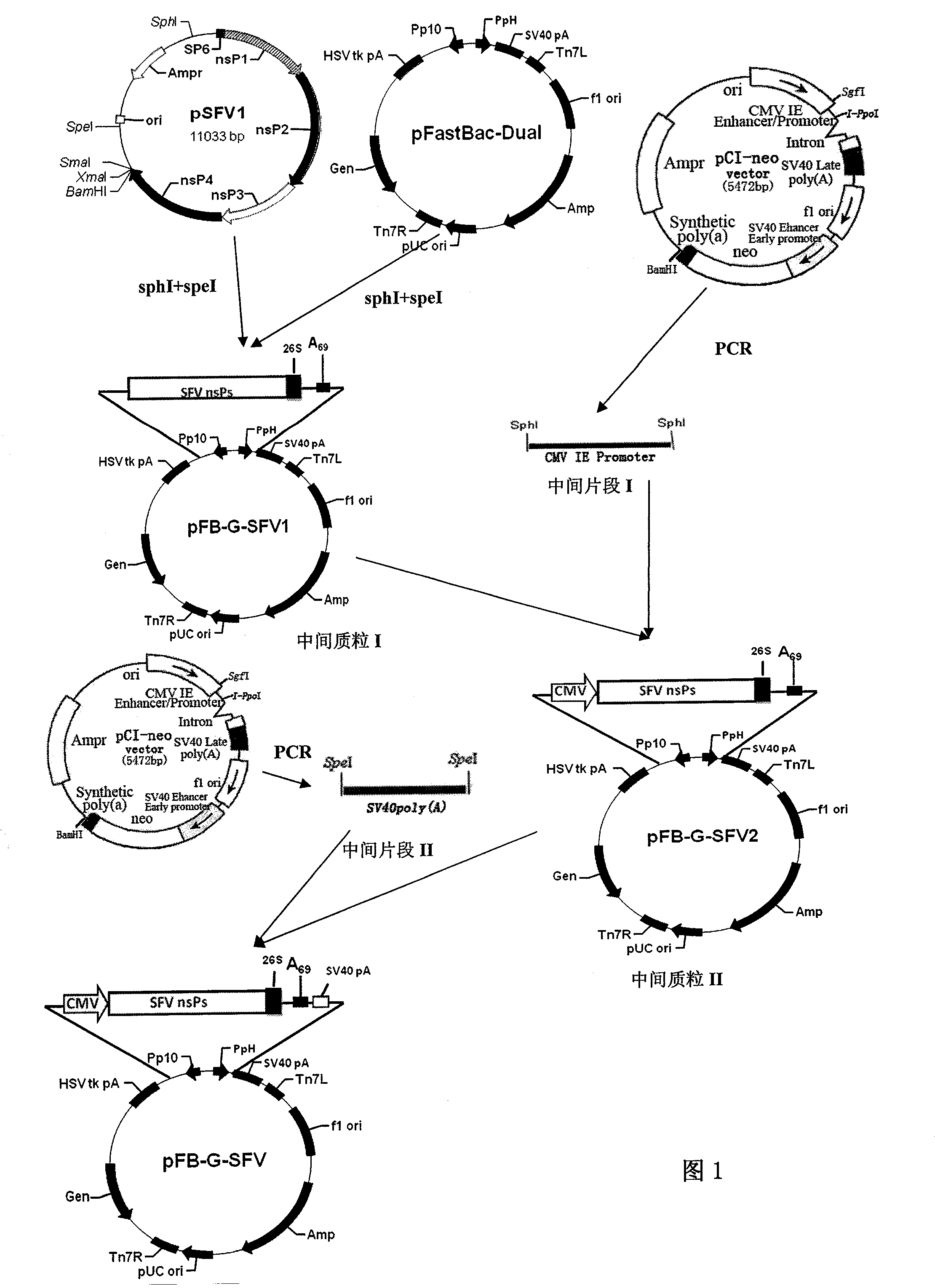
[0024] by figure 1 The shown pFastBac-Dual (purchased from Invitrogen Company) and pSFV1 (purchased from Invitrogen Company) are materials, according to figure 1 Shown flow process, construction said plasmid of the present invention, concrete steps are as follows:
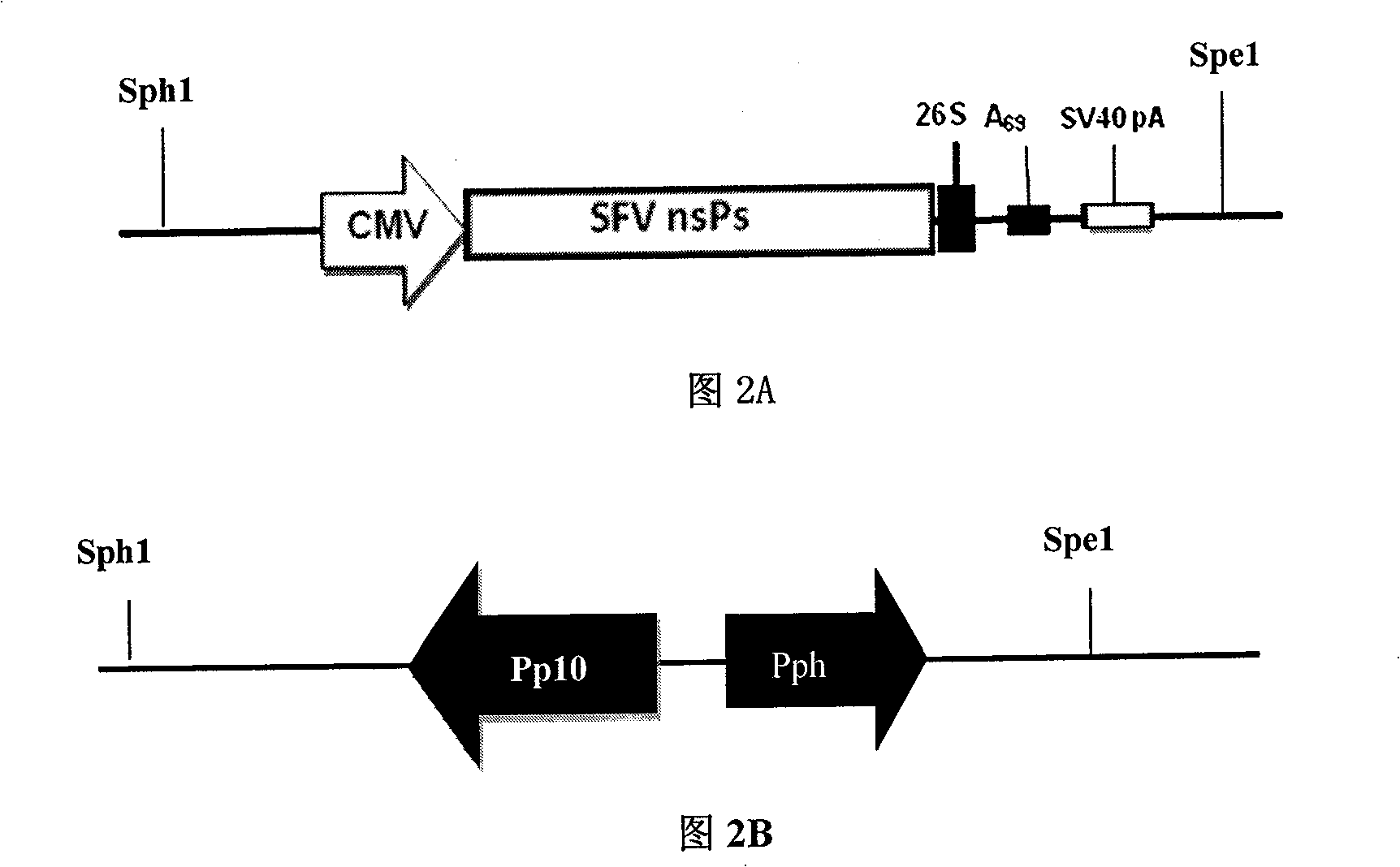
[0025] 1. The pFastBac-Dual and pSFV1 plasmids were digested with SphI and SpeI respectively, and the large fragment containing the backbone of the baculovirus transfer vector and the nonstructural protein nsPs1-4 of SFV and the linearized pFastBac-Dual were respectively recovered. The two fragments were ligated to obtain the intermediate plasmid I, namely pFB-G-SFV1;
[0026] 2. Use pCI-neo as a template to amplify the HCMV IE Enhancer / Promoter element with SphI restriction sites at both ends, such as figure 1 Middle Fragment I shown;
[0027] 3. Insert the HCMV IE Enhancer / Promoter element obtained in step 2 into the SphI site of the intermediate plasmid I to obtain the intermediate plasmid II, namely pFB-G...
Embodiment 2
[0031] 1. Construction of pFB-G-SFV-EGFP recombinant plasmid
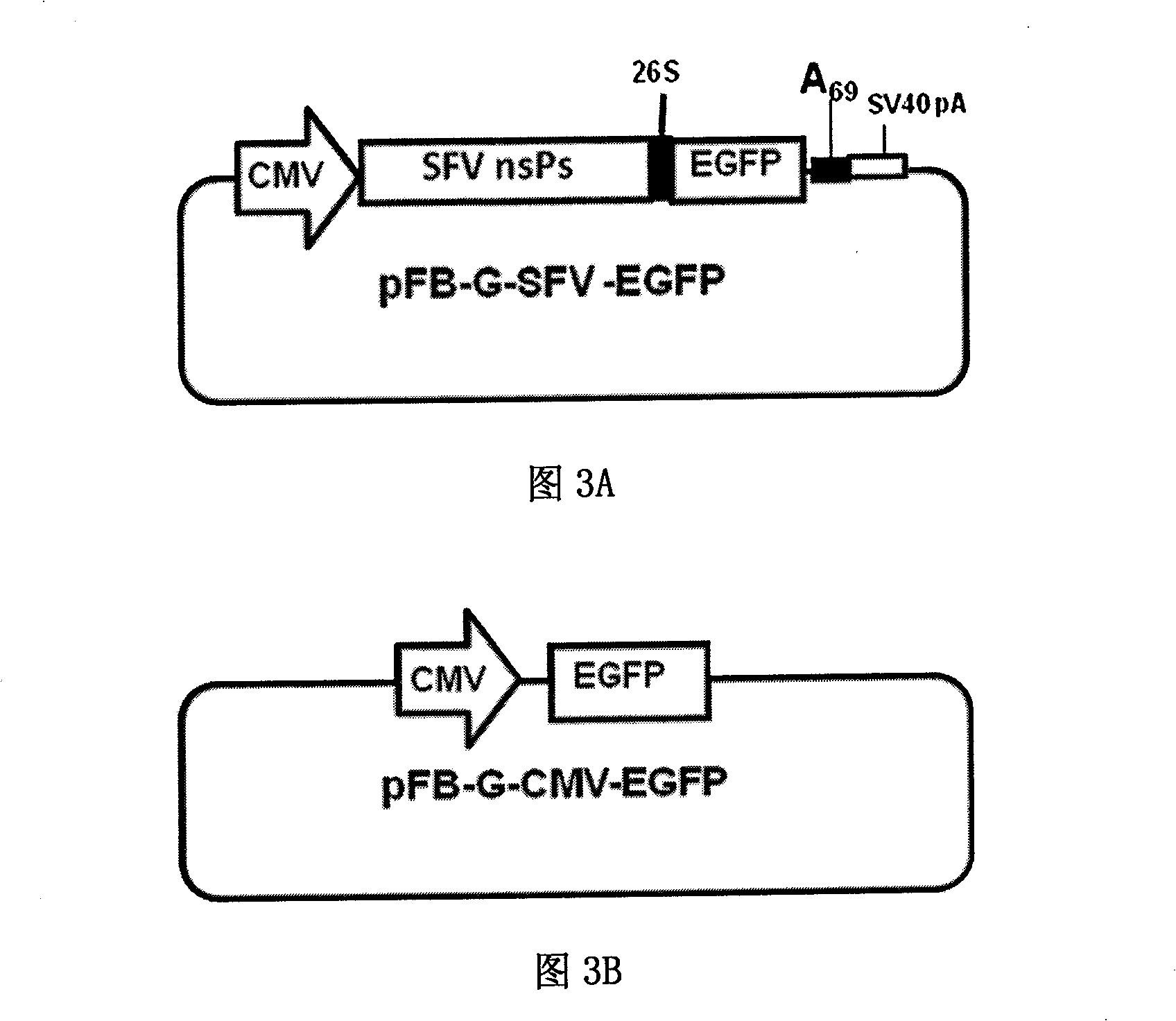
[0032] In this example, the fluorescent reporter gene EGFP (Genebank serial number: 1377915) will be used to directly and objectively evaluate whether the modified vector obtained in the present invention can correctly express foreign genes, and compare the modified plasmid with the currently commonly used baculovirus backbone Plasmids vary in transfection efficiency and expression levels. Using plasmid pEGFP-C1 (purchased from ClonTech Company) as a template to amplify the EGFP sequence (Genebank sequence number: 1377915) with BamHI and SmalI restriction sites at both ends, insert the plasmid pFB-G- obtained in Example 1 The BamHI and SmalI restriction sites of SFV were cut to obtain the recombinant plasmid pFB-G-SFV-EGFP. The results are attached image 3 As shown in A.
[0033] 2. Transposition of recombinant plasmid containing pFB-G-SFV-EGFP
[0034] 2.1 Take out DH10Bac TM Competent cells (purchased from ...
Embodiment 3( application Embodiment 1
[0061]1. In a six-well plate with 9×10 5 The density inoculation number of each cell / 2ml / hole is GDC010 Syrian hamster kidney cell line BHK-21 (purchased from the commercial cell material of the Chinese Type Culture Collection Center in Wuhan University, China, see http: / / www.bio-equip.com / showlequip.asp? equipid=14757&division=2604 ) cells, cultured at 37°C for 1 h to allow the cells to adhere to the wall.
[0062] 2, with Ca 2+ , Mg 2+ Phosphate buffered saline solution (PBS, purchased from Sigma Company) was used to wash the cells three times, and the baculovirus Bac-G-SFV-EGFP was inserted into the six-well plate cells at a dose of 50 multiplicity of infection (MOI=50). After infection for 4 hours at °C, the supernatant was discarded, and fresh DMEM containing 10% newborn bovine serum (purchased from Gibico) was added.
[0063] 3. Place the six-well plate in CO at 37°C 2 In the incubator, cultivate for 48 hours, and detect the expression of exogenous gene EGFP by flu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com