Method for producing piperacillin sodium tazobactam sodium compound injection
A technology of piperacillin sodium and tazobactam sodium, applied in the field of medicine, can solve the problems of large side effects and low purity of active ingredients, and achieve the effects of low cost, improved solubility and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
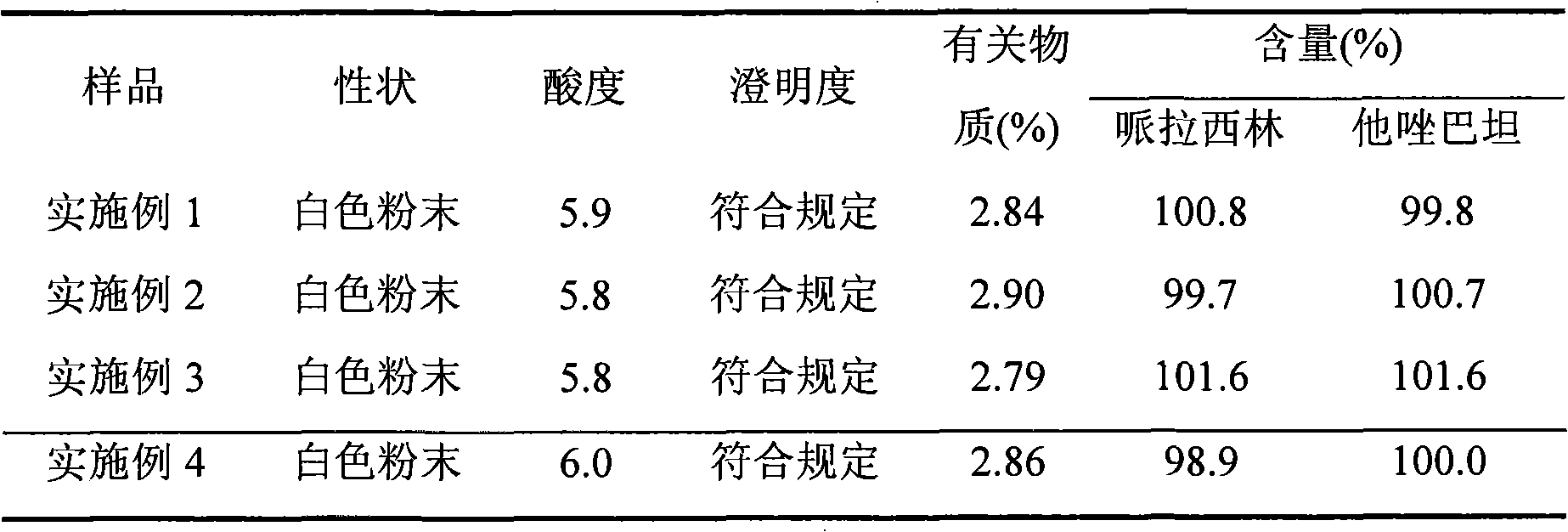
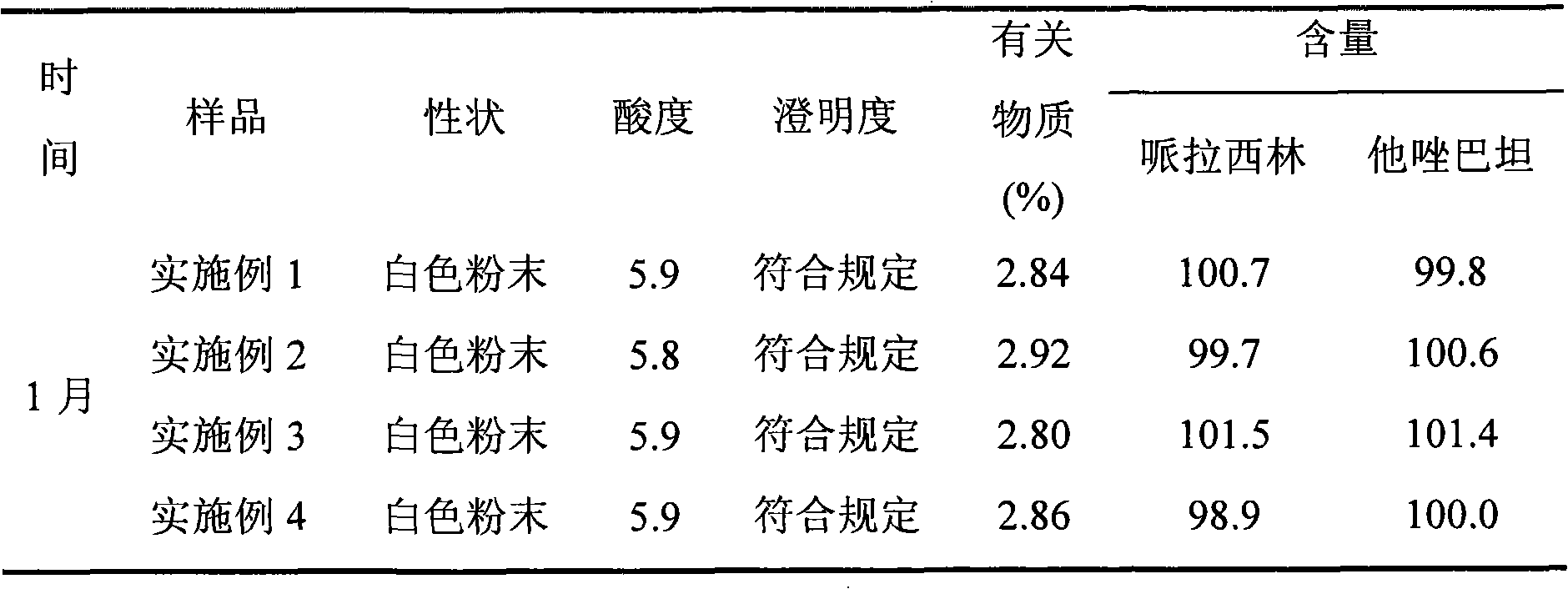
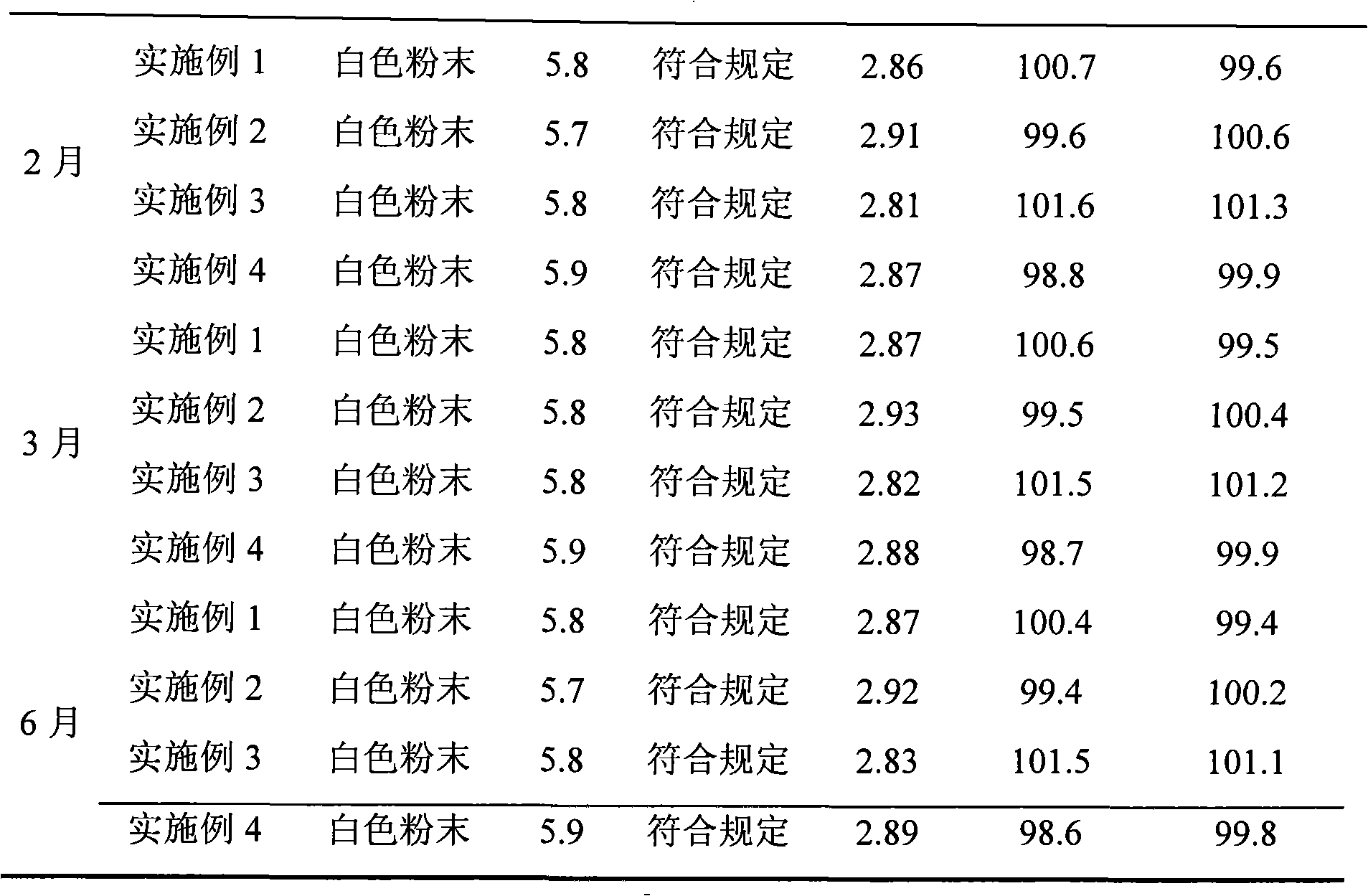
[0045]The piperacillin sodium raw material for injection is dissolved in water, and the resulting solution is subjected to gel chromatography. The packing of the chromatographic column used is Sephadex LH-20, which is eluted with 60%-95% ethanol solution, and the piperacillin sodium is collected The elution fraction with content not less than 90%. The resulting eluted fraction was chromatographed with BUCHI medium pressure preparative chromatography, and the filler of the reverse chromatography column used was C 18 Alkyl bonded phase silica gel. Elute with 60%-80% ethanol solution, collect the eluted fraction with piperacillin sodium content not less than 99%, recover the solvent, and freeze-dry to obtain high-purity piperacillin sodium raw material with a yield of 85.2%.
[0046] The raw material of tazobactam sodium for injection is dissolved in water, and the resulting solution is subjected to gel chromatography. The filler of the chromatographic column used is Sephadex LH...
Embodiment 2
[0049] The piperacillin sodium raw material for injection is dissolved in water, and the resulting solution is subjected to gel chromatography. The filler of the chromatographic column used is Sephadex LH-60, which is eluted with 60%-95% ethanol solution, and the piperacillin sodium is collected The elution fraction with content not less than 90%. The resulting eluted fraction was chromatographed with BUCHI medium pressure preparative chromatography, and the filler of the reverse chromatography column used was C 4 Alkyl bonded phase silica gel. Elute with 60%-80% ethanol solution, collect the eluted fraction with piperacillin sodium content not less than 99%, recover the solvent, and freeze-dry to obtain high-purity piperacillin sodium raw material with a yield of 81.0%.
[0050] Dissolve the raw material of tazobactam sodium for injection in water, and perform gel chromatography on the obtained solution. The filler of the chromatographic column used is Sephadex LH-60, and el...
Embodiment 3
[0053] The high-purity piperacillin sodium and tazobactam sodium raw materials prepared according to Example 1 were mixed in a weight ratio of 4:1, pulverized through a 100-mesh sieve, and packed under the condition of grade 100 in a sterile room to obtain final product Piperacillin sodium-tazobactam sodium compound powder injection, each unit powder injection contains 3.375g piperacillin sodium and tazobactam sodium.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com