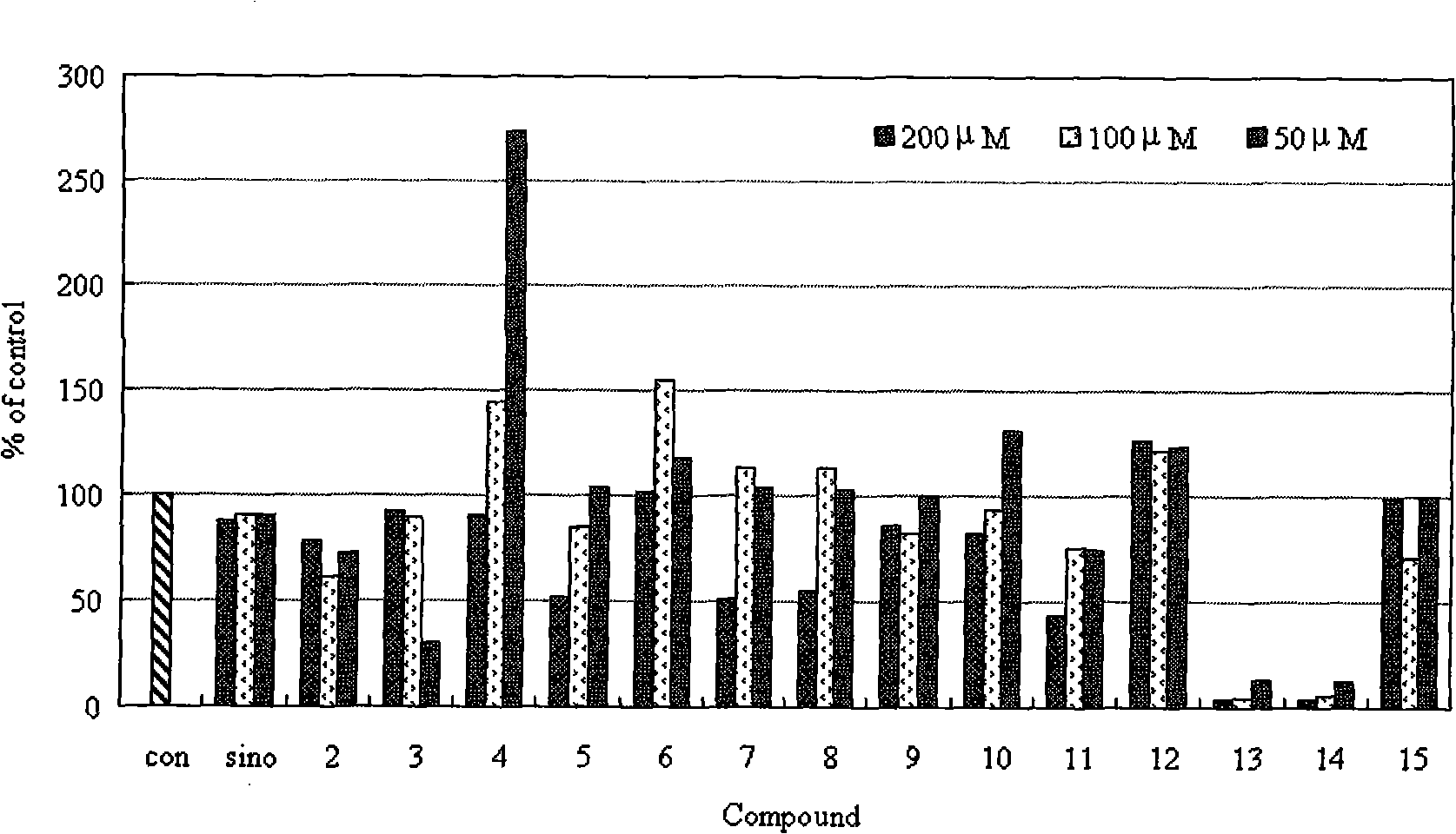
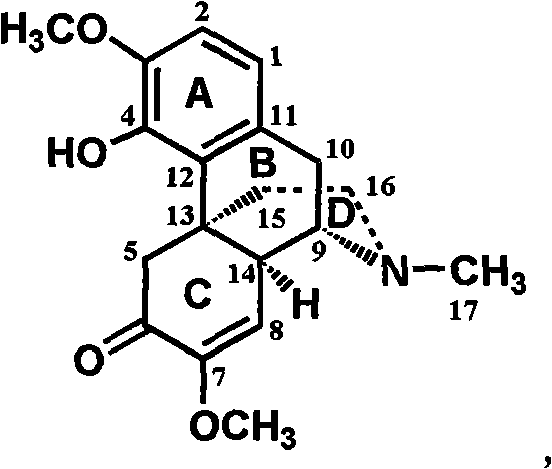
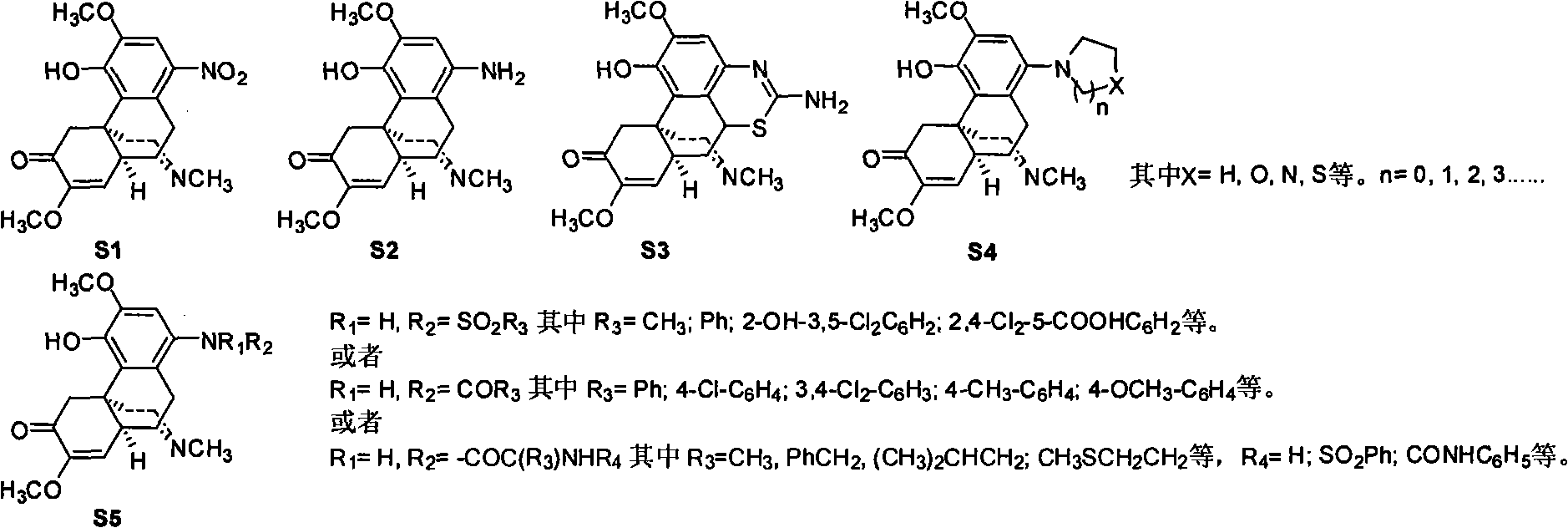
Sinomenine derivative, preparation method and application thereof
A technology of sinomenine and derivatives, applied in the field of polymer compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Synthesis of 1, 10-position nitrogen-sulfur six-membered heterocycle
[0077]
[0078]According to the above chemical formula, weigh compound 1: 550mg (1.6mmol) of 1-aminosinomenine, add 4.0ml KSCN (632mg, 2.4mmol) to the glacial acetic acid solution, stir and dissolve, slowly add Br 2 (96mg, 0.6mmol) in 1.5ml of glacial acetic acid solution. Stir at room temperature for 24 h, add 10% NaOH dropwise under ice bath to quench, adjust the pH value to 8.0, and use CHCl 3 Extraction, the obtained organic layer was washed with saturated brine, and then washed with anhydrous NaSO 4 Drying, concentration under reduced pressure, silica gel column separation (CH 3 Cl:CH 3 OH=9:1), the corresponding compound 2 was obtained with a yield of 59%.
[0079] Compound 2:
[0080] 1 H NMR (300MHz, CDCl 3 )δppm 8.45(s, 1H), 7.35(s, 1H), 6.30(s, 1H), 5.85(s, 1H, OH), 5.50(s, 1H), 4.24(d, 1H, J=15.9Hz) , 3.82(s, 3H), 3.29(m, 4H), 3.08(s, 1H), 2.56(m, 1H), 2.44(s, 3H), 2.40(s, 1H), ...
Embodiment 2
[0083] 1-position aminosulfonylation reaction
[0084]
[0085] According to the above chemical formula, weigh compound 1: 550mg (1.6mmol) of 1-aminosinomenine, dissolve in 3ml CH 2 Cl 2 , dropwise added Et 3 N 0.34ml (2.5mmol), add p-toluenesulfonyl chloride 381mg (2.0mmol) under ice-cooling, and stir for 5h under ice-cooling. Pour 30ml CH 2 Cl 2 extraction, the organic layer was washed with saturated brine, and then washed with anhydrous NaSO 4 Drying, concentration under reduced pressure, silica gel column separation (CH 3 Cl:CH 3 OH=9:1), the corresponding compound 3 was obtained with a yield of 52%.
[0086] Compound 3:
[0087] 1 H NMR (300MHz, CDCl 3 )δppm 7.58(d, 2H, J=6.6Hz), 7.25(d, 2H, J=6.6Hz), 6.39(s, 1H), 5.85(s, 1H, OH), 5.39(s, 1H), 4.30 (d, 1H, J=15.9Hz), 3.85(s, 1H), 3.62(s, 3H), 3.47(s, 3H), 3.20(brd, 1H), 3.01(brd, 1H), 2.78(m, 1H), 2.59-2.44(brd, 1H), 2.40(s, 3H), 2.36(s, 1H), 2.19(brd, 3H), 2.03-1.82(m, 4H).
[0088] 13C NMR (300MHz, CDCl ...
Embodiment 3
[0090] Amino acylation reaction at position 1
[0091]
[0092] According to the above chemical formula, weigh compound 1: 550mg (1.6mmol) of 1-aminosinomenine, dissolve in 3ml CH 2 Cl 2 , dropwise added Et 3 N 0.34ml (2.5mmol), add 313mg (2.0mmol) of benzoyl chloride under ice-cooling, and stir for 5h under ice-cooling. Pour 30ml CH 2 Cl 2 extraction, the organic layer was washed with saturated brine, and then washed with anhydrous NaSO 4 Drying, concentration under reduced pressure, silica gel column separation (CH 3 Cl:CH 3 OH=9:1), the corresponding compound 4 was obtained with a yield of 73%.
[0093] Compound 4:
[0094] 1 H NMR (300MHz, CDCl 3 )δppm 7.95(d, 2H, J=7.2Hz), 7.54(d, 1H, J=9.3Hz), 7.53(dd, 2H, J=7.2, 9.3Hz), 6.93(s, 1H), 5.49(s , 1H), 4.39(s, 1H, J=15.9Hz), 3.82(s, 3H), 3.61(brd, 1H), 3.51(s, 3H), 3.40(brd, 1H), 3.10-2.90(m, 4H), 2.65(s, 3H), 2.55-2.48(m, 2H), 2.21-2.05(m, 2H).
[0095] 13C NMR (300MHz, CDCl 3 )δ193.63,152.68,145.79,143.77,1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com