Cathode material for middle and low-temperature solid oxide fuel battery
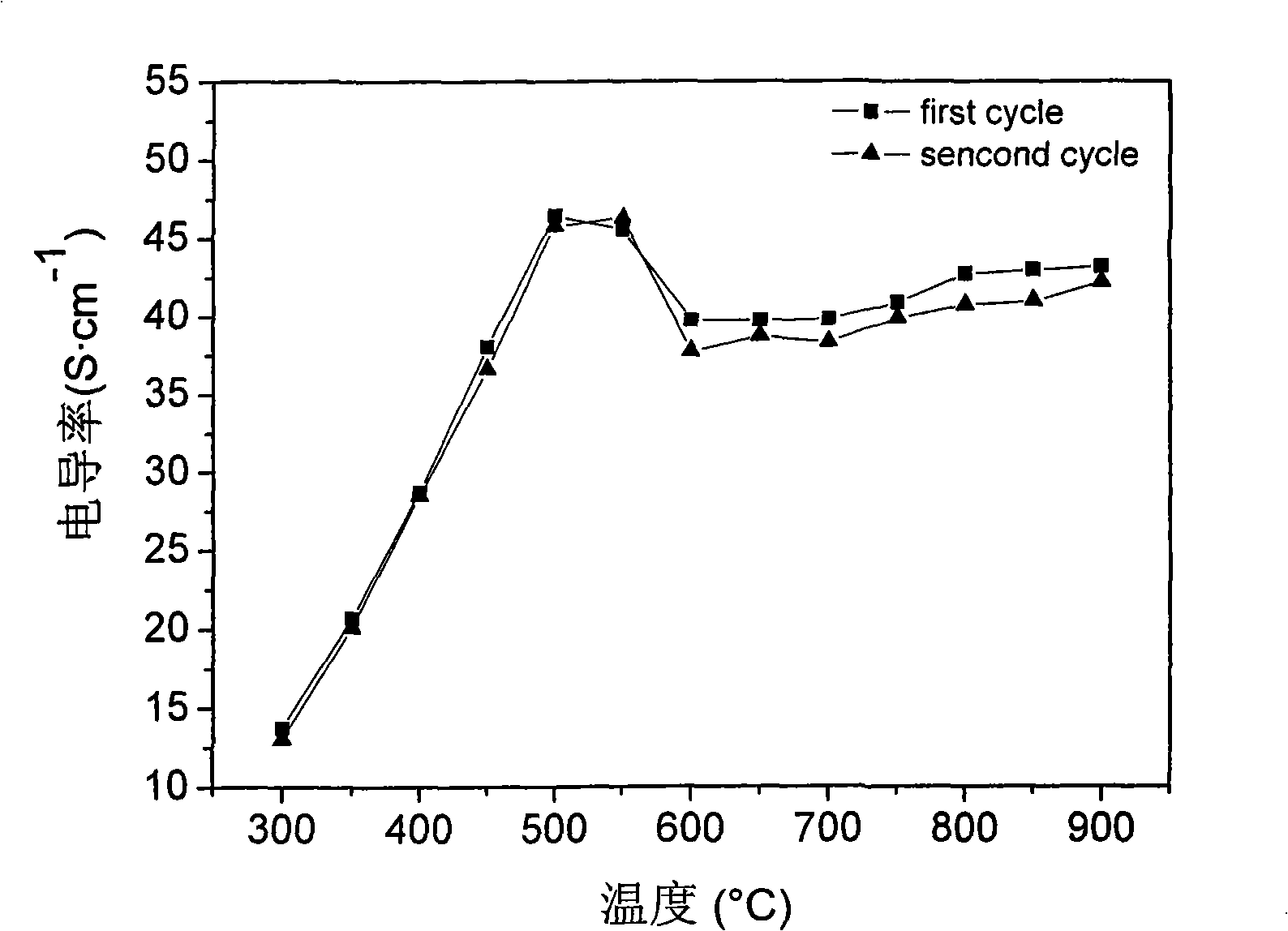
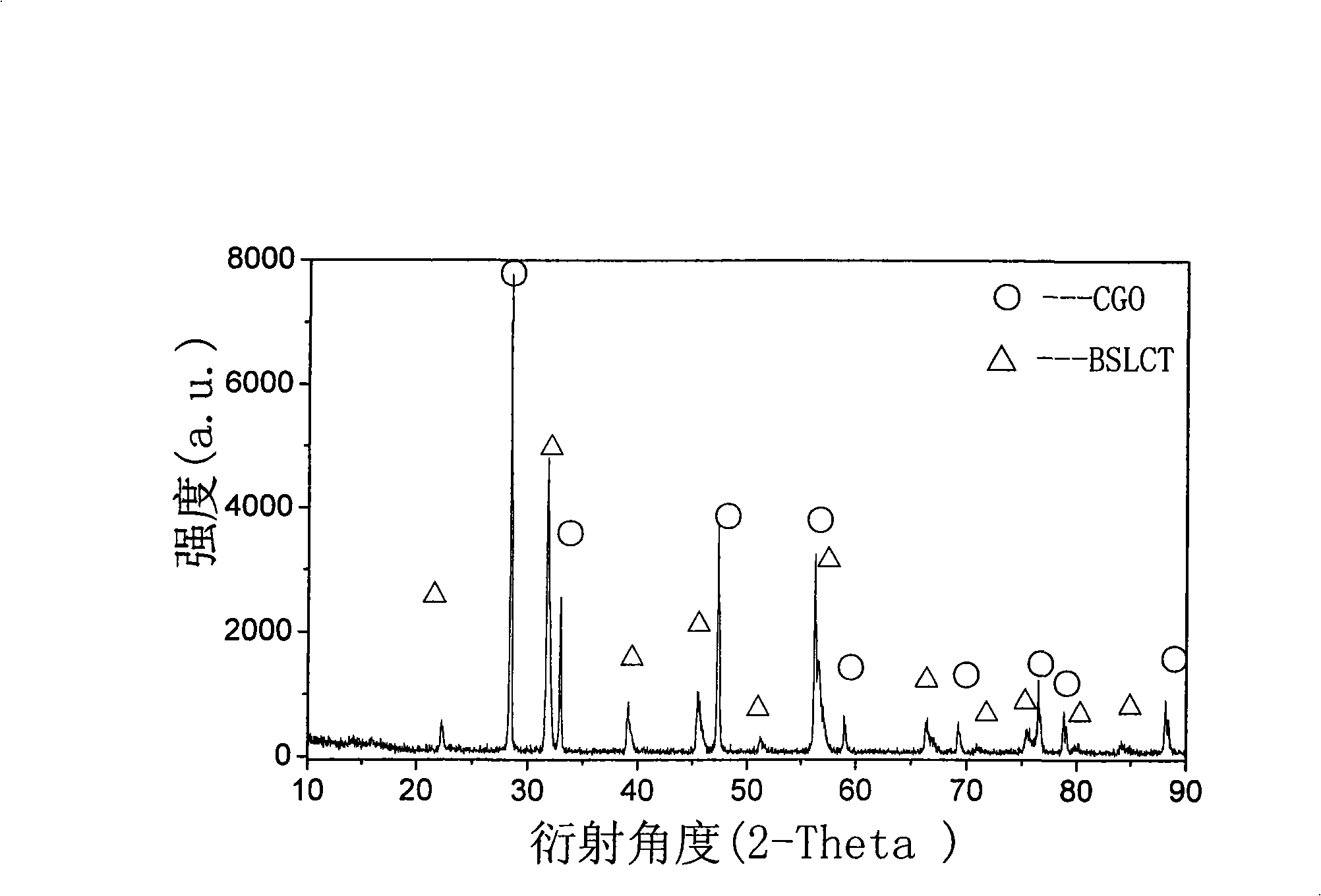
A fuel cell cathode, solid oxide technology, used in solid electrolyte fuel cells, fuel cells, fuel cell components, etc., can solve problems such as low electrical conductivity, and achieve good cell performance, good compatibility, and stable electrical conductivity. The effect of rate cycling performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment one: 0.05mol (Ba 0.6 Sr 0.4 ) 0.9 La 0.1 co 0.85 Ti 0.15 o 3-δ Synthesis, conductivity test and chemical stability test.
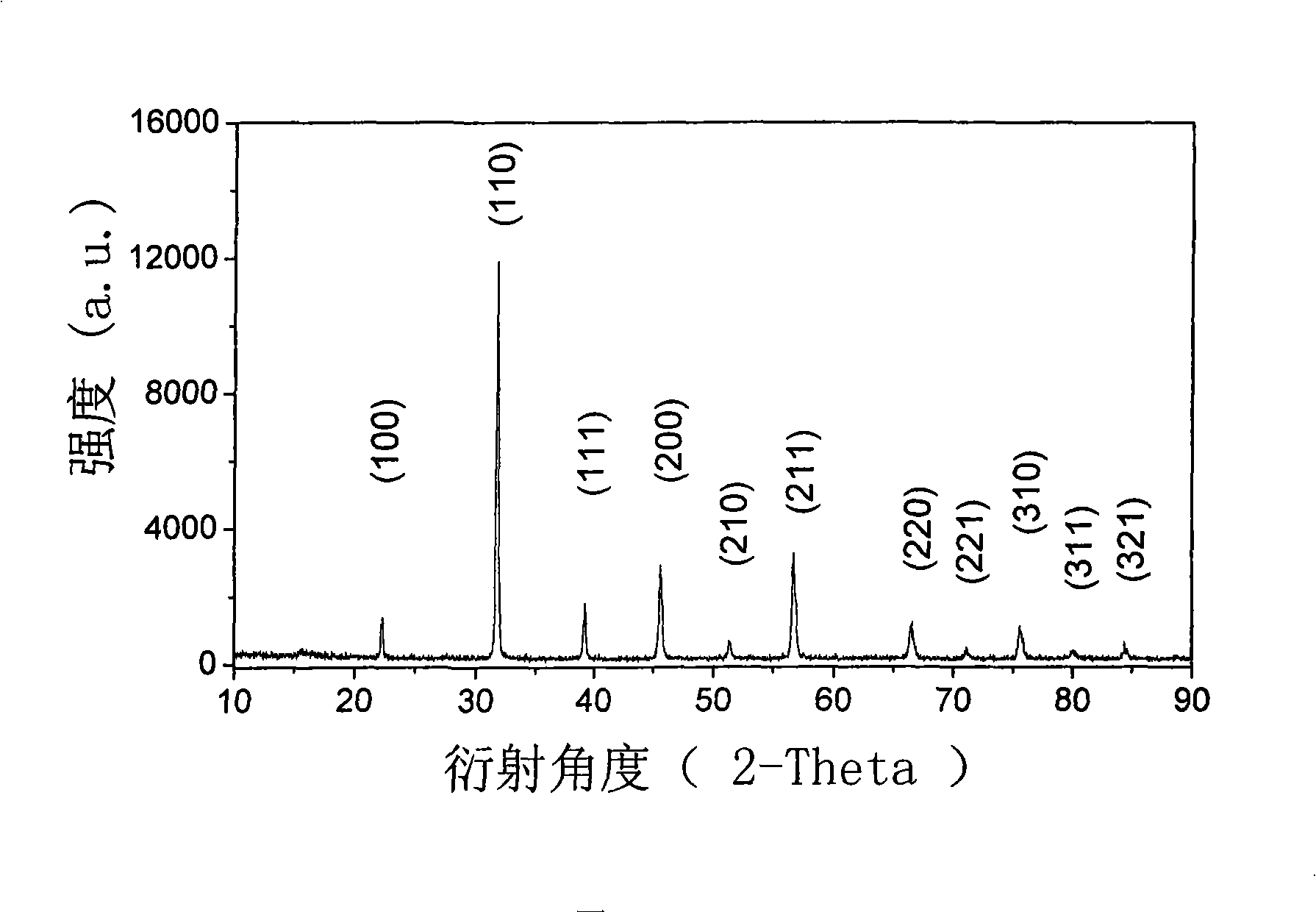
[0016] Weigh 5.328g BaCO 3 (analytical pure), 2.657g SrCO 3 (analytical pure), 0.8145g La 2 o 3 (analytical pure), 0.599g TiO 2 (analytical pure) and 10.586g C 4 h 6 CoO 4 4H 2O (analytically pure). Grind the above materials in a ball mill jar for 5 hours to make them evenly mixed, using agate balls as grinding media, and alcohol as a dispersant. The homogeneously mixed slurry was dried, and the dried material was calcined at 1100° C. for 10 hours to obtain the desired cathode material (Ba 0.6 Sr 0.4 ) 0.9 La 0.1 co 0.85 Ti 0.15 o 3-δ . The prepared powder was determined to be a cubic perovskite structure by XRD powder diffraction method, such as figure 1 shown.
[0017] After pre-burning the dried material at 900°C for 5 hours, grind and sieve (100 mesh), add 20% (volume ratio) carbon powder, 2% (volume ratio) PV...
Embodiment 2
[0020] Embodiment two: 0.05mol (Ba 0.6 Sr 0.4 ) 0.95 La 0.05 co 0.85 Ti 0.15 o 3-δ Synthesized by solid phase reaction method.
[0021] Weigh 5.624g BaCO 3 (analytically pure), 2.805g SrCO 3 (analytical pure), 0.407g La 2 o 3 (analytical pure), 0.599g TiO 2 (analytical pure) and 10.586g C 4 h 6 CoO 4 4H 2 O (analytically pure). Pour the above materials into a ball mill jar, use agate balls as a grinding medium, and alcohol as a dispersant. After mixing and milling for 5 hours, the uniformly mixed slurry is dried. The dried material was calcined at 1100°C for 12 hours to obtain a cathode material with a cubic perovskite structure (Ba 0.6 Sr 0.4 ) 0.95 La 0.05 co 0.85 Ti 0.15 o 3-δ . Sieve the powder synthesized at 1100°C (160 mesh), add 10% by mass fraction of soluble starch and 1% by mass fraction of ethyl cellulose to 1g of cathode material, and finally add 1ml of deionized water, mix well and use The screen printing method is evenly coated on the surf...
Embodiment 3
[0022] Embodiment three: 0.05mol (Ba 0.6 Sr 0.4 ) 0.85 La 0.15 co 0.8 Ti 0.2 o 3-δ Synthesized by solid phase reaction method.
[0023] Weigh 5.032g BaCO 3 (analytical pure), 2.510g SrCO 3 (analytical pure), 1.222g La 2 o 3 (analytical pure), 0.599gTiO 2 (analytical pure) and 10.586g C 4 h 6 CoO 4 4H 2 O (analytically pure). Pour the above materials into a ball mill jar, use agate balls as a grinding medium, and alcohol as a dispersant. After mixing and milling for 5 hours, the uniformly mixed slurry is dried. The dried material was calcined at 1100°C for 10 hours to obtain a cathode material with a cubic perovskite structure (Ba 0.6 Sr 0.4 ) 0.85 La 0.15 co 0.85 Ti 0.15 o 3-δ .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com