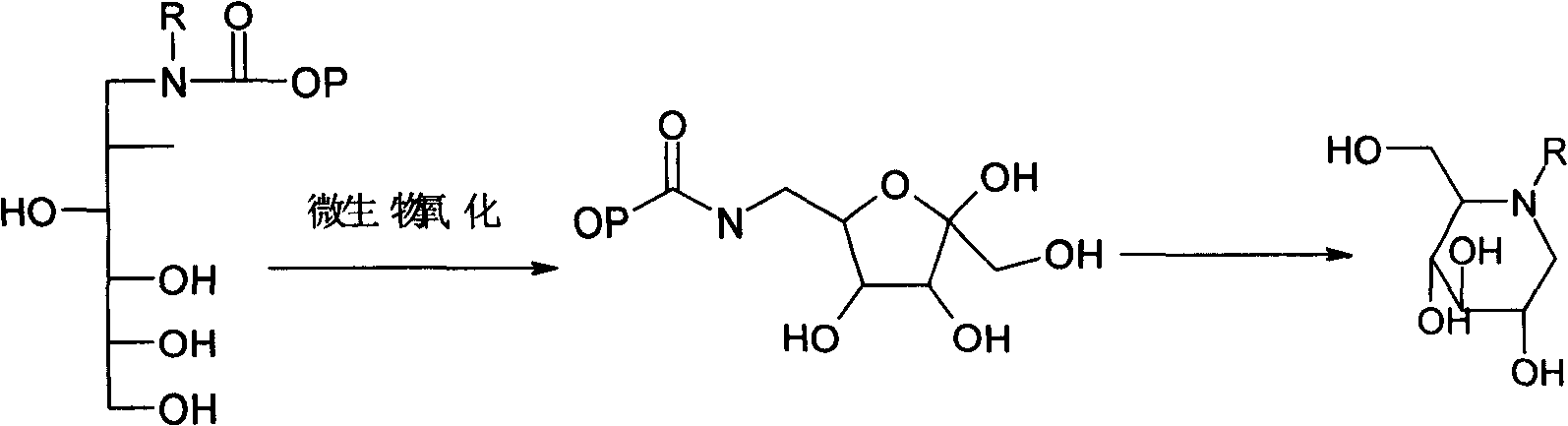
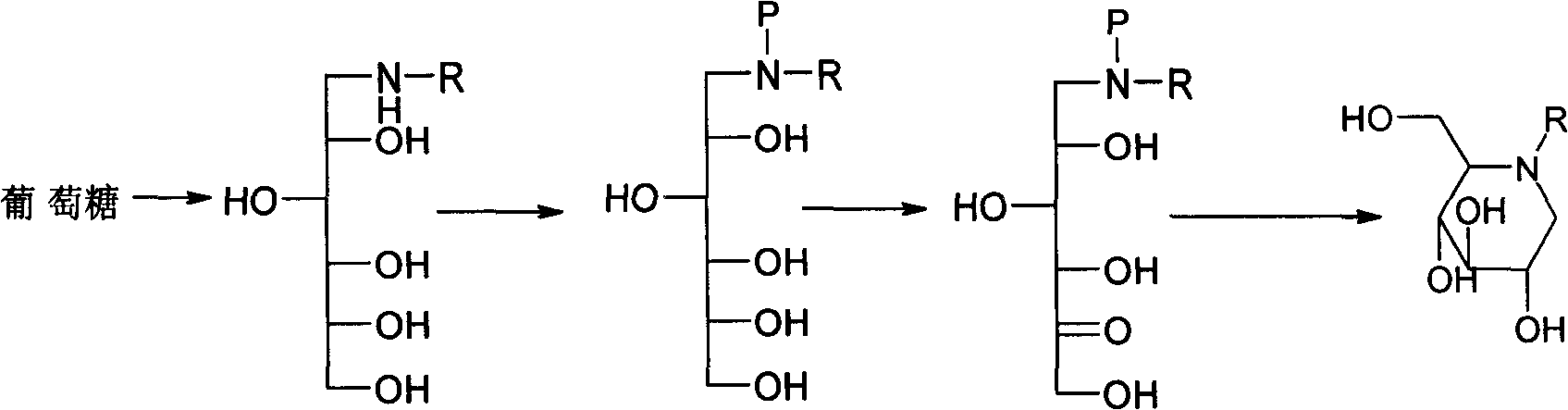
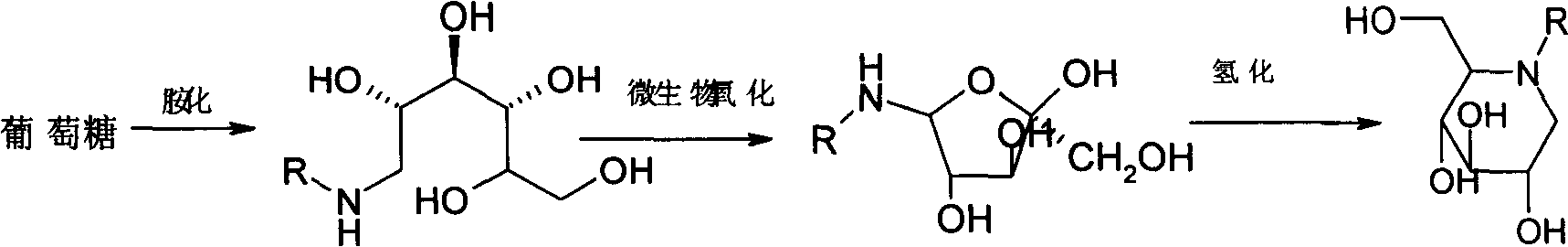
Method for preparing miglitol midbody N-substituted-1-deoxidization nojirimycin derivative
A technology of deoxynojirimycin and miglitol, applied in the direction of microorganism-based methods, biochemical equipment and methods, microorganisms, etc., can solve the problem of large differences between bacterial batches, high cost of biotransformation, and inability to adapt to large-scale industrialization. Production and other issues, to achieve the effects of reduced production costs, short processing time, and excellent slow-release performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A. Bacterial liquid preparation: Take 10000L of the fermentation liquid of Glucose oxidase bacteria cultured to the end of logarithmic growth, remove the fermentation liquid by microfiltration with a ceramic membrane with a pore size of 0.2 μm, and use 0.3% MgSO 4 The solution was top-washed until the total sugar was 0.065%, the amino nitrogen was 2.8mg / 100ml, the temperature was controlled at 25±1°C, the pressure was 0.03MPa, and the pH was 5.0 to obtain 100L of bacterial solution.
[0042] B, conversion production: drop into conversion substrate N-(2-hydroxyethyl)-glucosamine 100kg and dissolve with purified water in 5000L fermenter, make conversion substrate final concentration be 10%, adjust pH value to be 6.0, add 0.32% MgSO 4 , adding the bacterial solution, so that the weight ratio of the transformed substrate to the bacterial body is 1:1, the automatic control transformation temperature is 24±1°C, the automatic control transformation pH value is 6.0, and the dis...
Embodiment 2
[0047] A. Bacterial solution preparation: take 10000L of gluconoxidase fermentation broth cultivated to the end of logarithmic growth, remove the fermentation broth by microfiltration with a ceramic membrane with a pore size of 0.05 μm, and use 0.6% MgSO 4 The solution was top-washed until the total sugar was 0.134%, the amino nitrogen was 5.6mg / 100ml, the temperature was controlled at 20°C, the pressure was 0.04MPa, and the pH was 7.0 to obtain 100L of bacterial solution.
[0048] B, conversion production: put into 5000L fermentor and put into conversion substrate N-(2-hydroxyethyl)-glucosamine 100kg and dissolve with purified water, make conversion substrate final concentration be 10%, adjust pH value to be 6.5, add 0.6% MgSO 4 , add the bacterial solution, the weight ratio of the transformation substrate to the bacteria is 1:3, the transformation temperature is manually controlled to be 6±1°C, the pH value of the transformation is manually controlled to be 6.5, and the diss...
Embodiment 3
[0053] A. Bacterial solution preparation: Take 10000L of glucose oxidative bacteria fermentation broth cultivated to the end of logarithmic growth, filter and remove the fermentation broth with a polytetrafluoroethylene membrane with a pore size of 1.0 μm, and wash with deionized water until the total sugar is 0.98%. Amino nitrogen was 47.6mg / 100ml, the temperature was controlled at 15°C, the pressure was 0.03Mpa, and the pH was 6.0 throughout the whole process to obtain 100L of bacterial liquid.
[0054] B, transformation production: put into 5000L fermentor and put into transformation substrate N-(2-hydroxyethyl)-glucosamine 100kg and dissolve with purified water, make transformation substrate final concentration be 10%, adjust pH value to be 4.0, add 0.32% MgSO 4 , add the bacterial solution, the weight ratio of the transformation substrate to the bacteria is 1:2, the automatic control transformation temperature is 10±1°C, the automatic control transformation pH value is 4....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com