Electrolytic apparatus for regenerating waste acid produced by acid cleaning of stainless steel, regeneration method therefor
An electrolysis device, stainless steel technology, applied in the field of electrolysis devices for the regeneration of waste acid after pickling of stainless steel, can solve the problems of unclear application prospects, secondary pollution, environmental pollution, etc., and achieve improved sample processing speed and efficiency, convenient disassembly, Effects of Overcoming Limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Spent acid regeneration electrolysis device
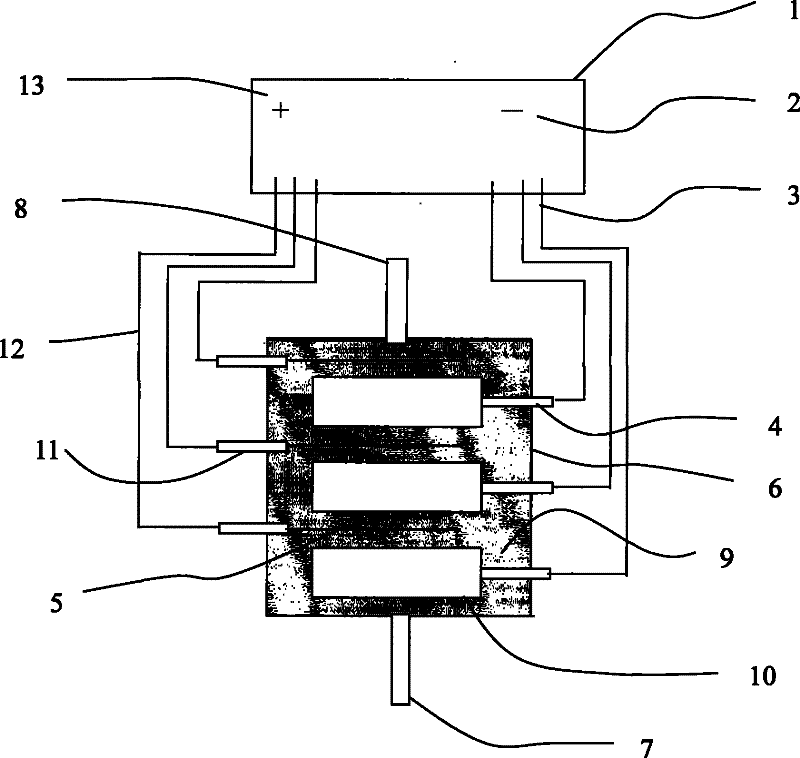
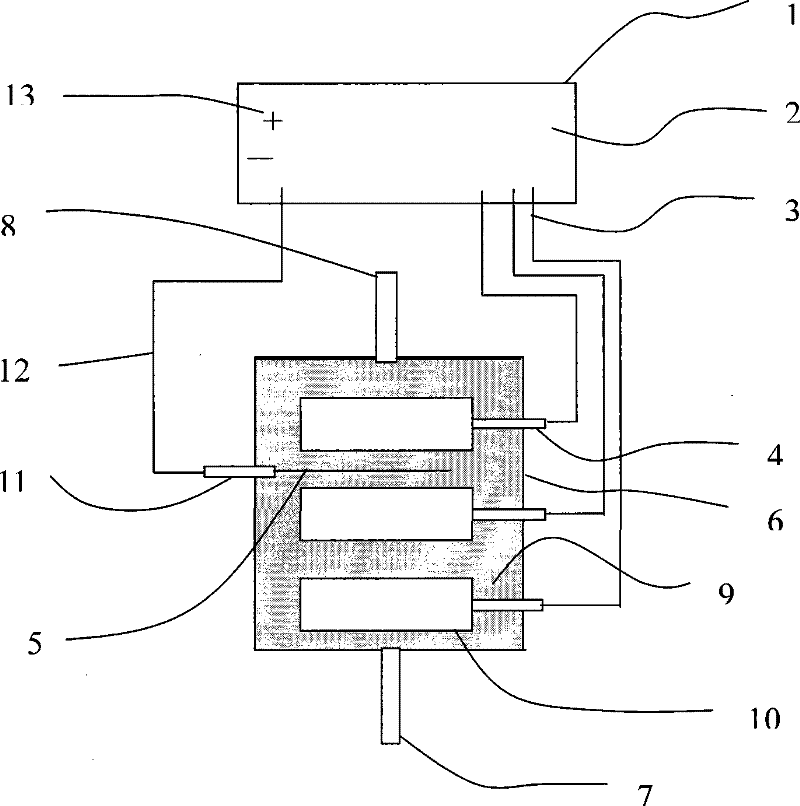
[0025] Waste acid electrolysis treatment device such as figure 1 As shown, the power supply 1 is a multiple input and output constant voltage power supply. The negative output terminal 2 of the power supply is connected to the cathode input terminal 4 of the electrolytic cell by a wire 3, and is connected to the cathode electrode 10 of the electrolytic cell. The electrode is cylindrical and enlarged. The electrolysis reaction area, the electrolytic cell 6 is a sealed cup-shaped container, with sample inlet 7 and sample outlet 8 at both ends, which is convenient for sample entry and product output. The anode electrode 5 is in the shape of a wire or sheet, and is connected to the anode of the electrolytic cell. The access terminal 11 is connected, and the entire flow path is finally connected to the positive output terminal 13 of the power supply through a wire 12. Add the waste acid 9 after stainless steel pickling in ...
Embodiment 2
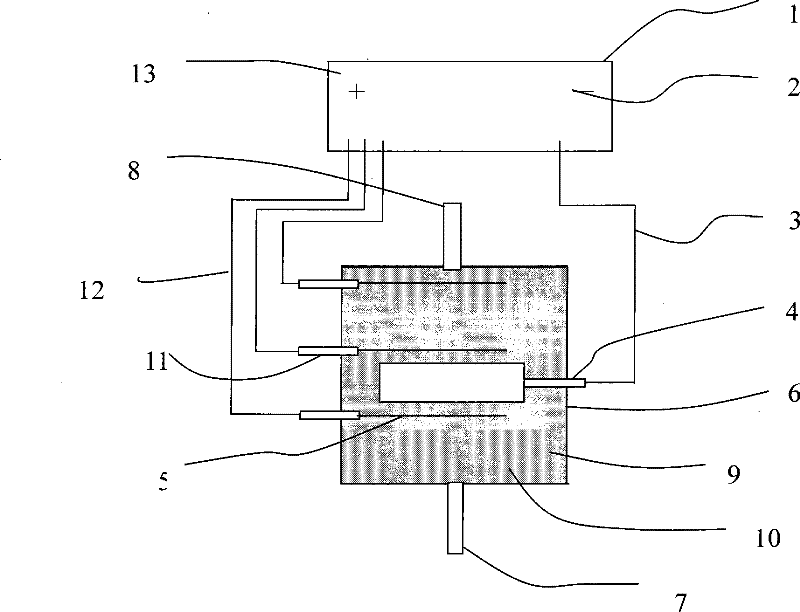
[0027] (1) such as Figure 4 As shown, a single columnar graphite cathode and filamentary platinum anode are used. The electrolyte is a waste acid solution A after pickling in a large stainless steel plant in my country. The main ion concentrations are respectively: iron ion concentration 62g / L, nickel ion concentration 1.5g / L , The chromium ion concentration is 4g / L, the fluoride ion concentration is 1.5mol / L, electrolyzed at a constant voltage of 3.5V for 15 hours to obtain solution A1;
[0028] (2) Pass the solution A1 through a flame atomic absorption instrument, and use the flame method or the graphite furnace method to measure the content of metal ions such as Fe, Ni, and Cr; use a fluoride ion selective electrode to measure the fluoride ion content in it, and calculate the electrolytic ion removal efficiency.
Embodiment 3
[0030] (1) Weigh 10g of anhydrous sodium sulfate, dissolve it with water and dilute it to 200mL to obtain catalyst S;
[0031] (2) such as Figure 4 As shown, using a single columnar graphite cathode and a filamentary platinum anode, the spent acid solution A is electrolyzed at a constant voltage of 3.5V, catalyst S is added, and the electrolysis time is 15 hours to obtain solution A2;
[0032] Step 3 is the same as step (2) in Example 2 to calculate the electrolytic metal ion removal efficiency.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com