Process for separating and utilizing mixing components of o-, m-, and p-nitrochlorobenzene
A technology of p-nitrochlorobenzene and o-nitrochlorobenzene, which is applied in the field of green and sustainable development separation and utilization engineering, can solve the problems of large investment, high cost, and secondary pollution, and achieve energy saving and consumption reduction, environmental pollution, and easy Separation and purification, the effect of high use value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
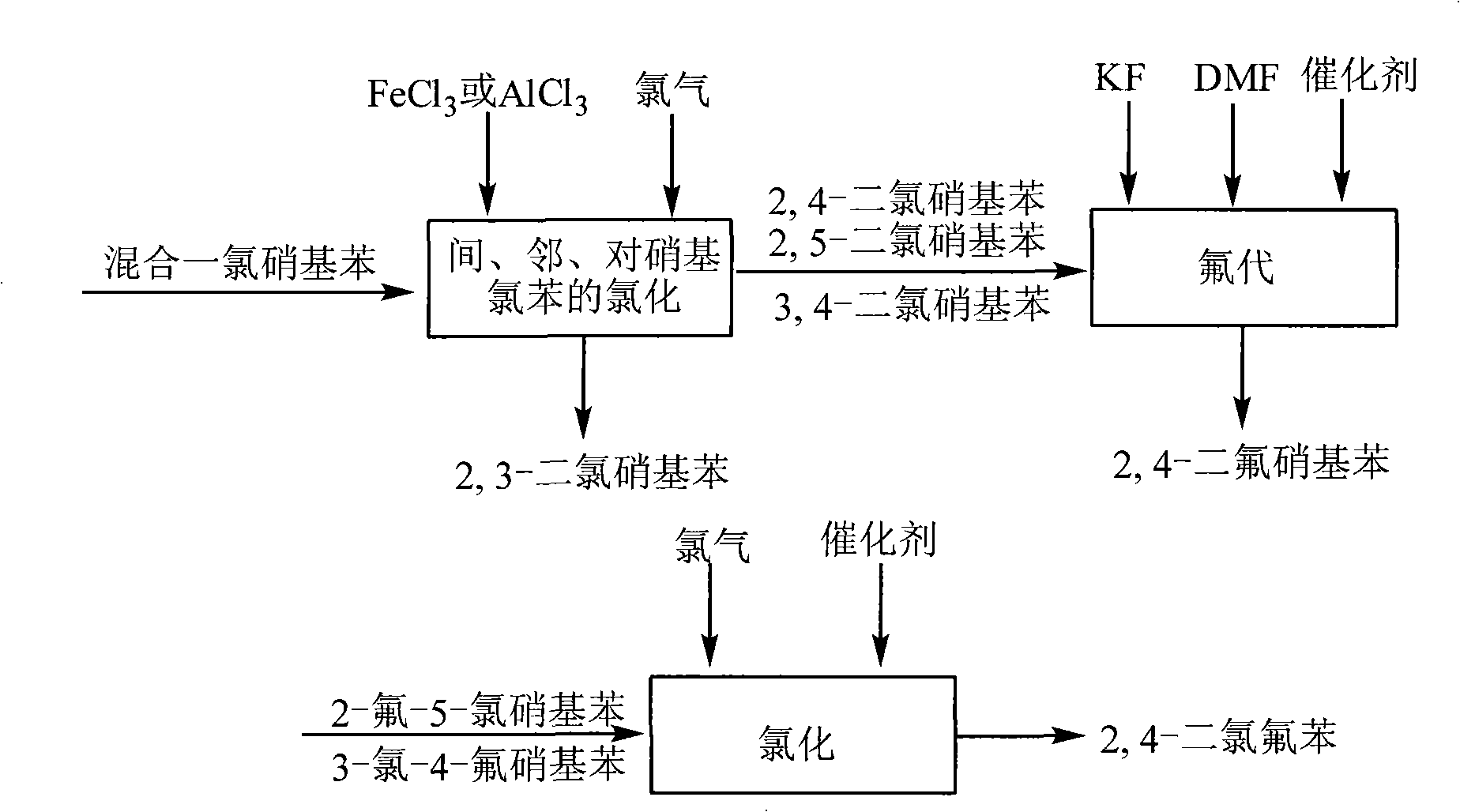
[0030] Add 157.5 kg of industrial production residues of p- and o-nitrochlorobenzene in the reaction kettle, add 1.58 kg of aluminum trichloride, heat to 80 ° C, and pass 15.8 kg of chlorine gas for about 8 hours for chlorination. Return the unchlorinated monochloronitrobenzene compound for rechlorination, and the product produced by chlorination is mixed dichloronitrobenzene: 3,4-dichloronitrobenzene, 2,5-dichloronitrobenzene, 2 , 4-dichloronitrobenzene, 2,3-dichloronitrobenzene. Then according to the difference in boiling point, 2.4 kg of 2,3-dichloronitrobenzene was separated by a rectification tower, and its purity was ≥99.5% as detected by gas chromatography, with a melting point of 62°C and a yield of about 1.3%; simultaneously, 2,4-dichloronitrobenzene was obtained. The mixture of chloronitrobenzene, 2,5-dichloronitrobenzene and 3,4-dichloronitrobenzene was 45.6 kg, and the yield was about 23.8%.
Embodiment 2
[0032] Add 157.5 kg of industrial production residues of p- and o-nitrochlorobenzene into the reaction kettle, add 3.95 kg of aluminum trichloride, heat to 95 ° C, and pass 60 kg of chlorine gas for about 9 hours for chlorination. Return the unchlorinated monochloronitrobenzene compound for rechlorination, and the product produced by chlorination is mixed dichloronitrobenzene: 3,4-dichloronitrobenzene, 2,5-dichloronitrobenzene, 2 , 4-dichloronitrobenzene, 2,3-dichloronitrobenzene. Then, according to the difference in boiling point, 7.2 kg of 2,3-dichloronitrobenzene was separated by a rectifying tower, and its purity was detected by gas chromatography to be ≥99.5%, with a melting point of 62°C and a yield of about 3.8%; at the same time, 2,4-dichloronitrobenzene was obtained. The mixture of chloronitrobenzene, 2,5-dichloronitrobenzene and 3,4-dichloronitrobenzene was 129.6 kg, and the yield was about 67.5%.
Embodiment 3
[0034] Add 157.5 kg of industrial production residues of p- and o-nitrochlorobenzene into the reaction kettle, add 7.87 kg of aluminum trichloride, heat to 105 ° C, and pass 157.5 kg of chlorine gas for about 9 hours for chlorination. Return the unchlorinated monochloronitrobenzene compound for rechlorination, and the product produced by chlorination is mixed dichloronitrobenzene: 3,4-dichloronitrobenzene, 2,5-dichloronitrobenzene, 2 , 4-dichloronitrobenzene, 2,3-dichloronitrobenzene. Then, according to the difference in boiling point, 15.4 kg of 2,3-dichloronitrobenzene was separated by a rectification tower, and its purity was ≥99.5% as detected by gas chromatography, with a melting point of 62°C and a yield of about 8%; at the same time, 2,4-dichloronitrobenzene was obtained. The mixture of chloronitrobenzene, 2,5-dichloronitrobenzene and 3,4-dichloronitrobenzene was 176.6 kg, and the yield was about 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com