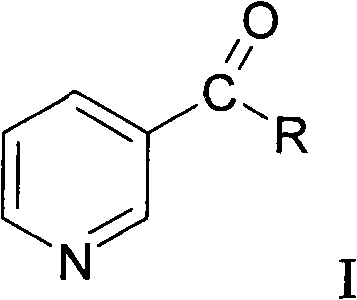
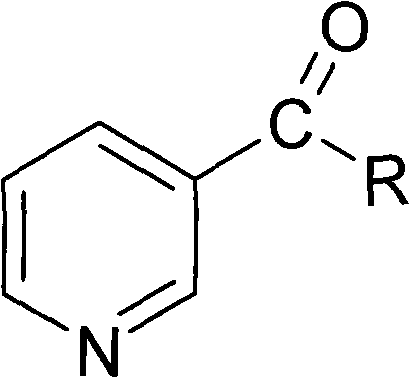
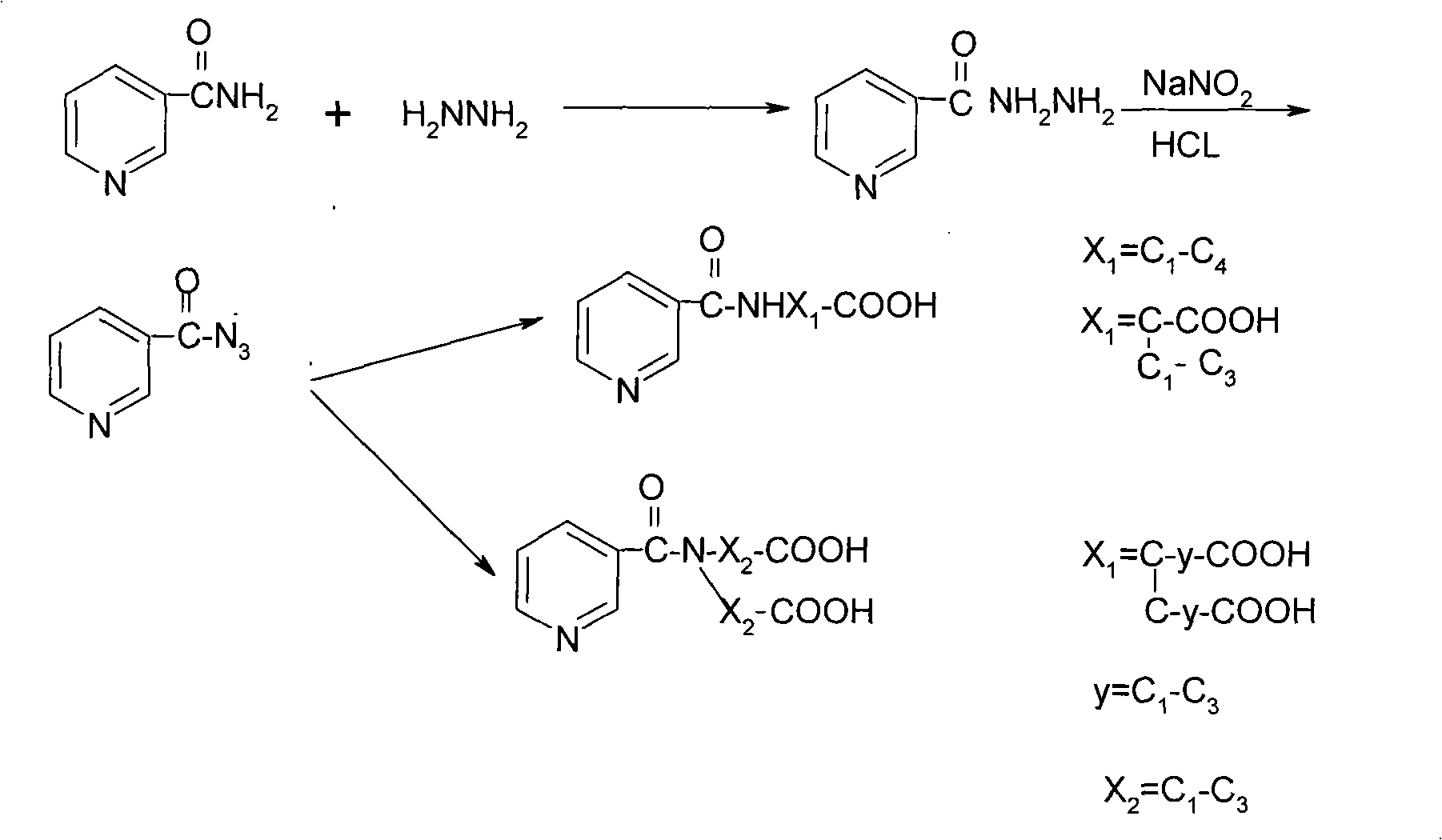
Method for synthesizing nicotinyl amino acids and sensitizing effect thereof to tumor radiotherapy
A technology of nicotinyl amino acid and compound, which is applied in the field of medicine, can solve the problems of limiting tumors and low radiation sensitivity, and achieve the effects of simple operation, good purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0049] Nicotinic acid hydrazide 18.7 (130mM) was dissolved in 122.2ml of 13% hydrochloric acid solution, and NaNO was added dropwise at low temperature 2 18.2g (260mM) in the solution of 45ml of water, after dripping, continue to react for 1.5 hours, the reaction mixture is washed with Na 2 CO 3 Adjust the pH to 7, extract with ether (100ml×4), wash the organic layer with water, CaCl 2 Drying and removal of solvent yielded 16.6 g of product, (86%). ,
example 2
[0051] Synthesis of N-Nicotinyliminodiacetic Acid
[0052] Add 2.3g (15.5mM) of nicotinyl azide to a solution of 2.1g (15.5mM) of iminodiacetic acid in 16ml of 1N sodium hydroxide, stir for 12 hours, then neutralize with hydrochloric acid until the pH reaches 3-4, and concentrate by rotation The reaction solution reaches a small volume (PH 3-4), and white crystals are precipitated and recrystallized with 80% methanol, mp 202-203°C.
[0053] 1HNMR (DMSO-d6), δ(ppm) 4.10(d, 4H, -CH2-, -CH2-), 7.48((dd, 1H, H5), 7.73(dd, 1H, H4), 8.50(dd, 1H , H6), 8.66 (q, 1H, H2).
example 3
[0055] 2-Nicotinamide butyric acid
[0056] Add 3.1g (21mM) of nicotinoyl azide powder to 2.16g (21mM) of DL-2-aminobutyric acid in 21ml of 1NNaOH solution in portions. After the addition is complete, continue to stir for 12 hours, rotate and concentrate to obtain a viscous substance, add dropwise Adjust the pH to 4 with 2N HCl, and 3.6 g (82%) of a white precipitate precipitated, mp 212-214°C, and recrystallized from ethanol.
[0057] 1HNMR (DMSO-d6) δppm0.968 (t, 3H, CH3), 1.810 (m, 2H, CH2), 4.317 (m, 1H, -CH-), 7.526 (dd, 1H, C53), 8.229 (m, 1H, C42), 8.727 (m, 1H, H6'), 8.821 (d, 1H, C21), 9.039 (d, 1H, NH) 12.666 (S, 1H, OH), Anal C 11 h 12 N 2 o 3 C, H, N.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com