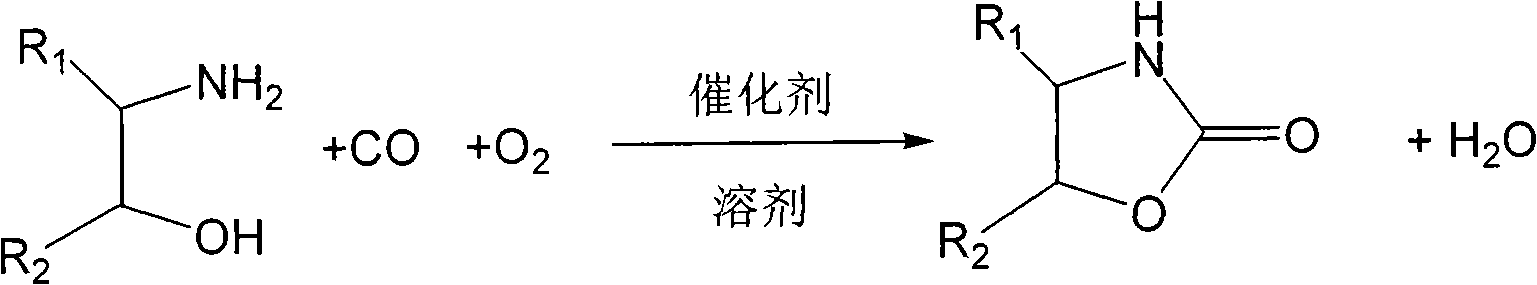
Method for synthesizing oxazoline-2-ketone
A technology for synthesizing oxazoline and oxazoline, applied in the direction of organic chemistry and the like, can solve problems such as difficult separation of catalyst and product, and achieve the effects of being beneficial to large-scale industrial production, reducing the burden of three-waste treatment, and low technological difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 10ml of acetonitrile, 0.61g (10mmol) of ethanolamine, 0.0395g (0.5mmol) of Se into the three-necked flask, put it into an oil bath heated to 30C, and stir the reaction with carbon monoxide for about 15 minutes. Oxygen was introduced at a certain rate, and the reaction was stirred for 8h. Stop CO, continue to pass through oxygen to oxidize for 2 hours, filter and recover the catalyst, evaporate the solvent in vacuo to obtain a crude product, after recrystallization and purification, weigh 0.84g of the product oxazolin-2-one, and the first single-pass yield is 93 % (calculated as ethanolamine).
Embodiment 2
[0028] The organic solvent is dimethylformamide, and the experimental method and steps are the same as those in Example 1, and the actual yield per pass for the first time is 85% (calculated by ethanolamine).
Embodiment 3
[0030] The organic solvent is pyridine, and the experimental method and steps are the same as those in Example 1, and the actual yield per pass for the first time is 93% (calculated by ethanolamine).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com