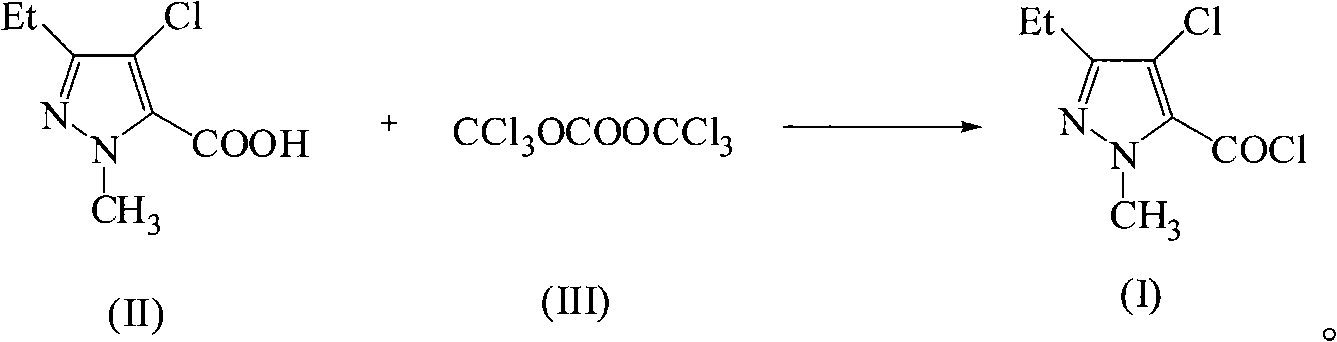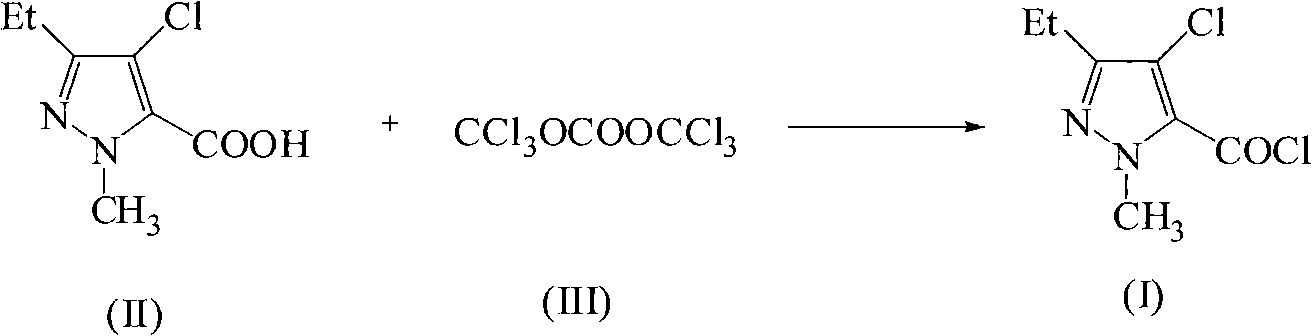Green synthetic method for methyl-3-ethyl-4-chlorin-5-pyrazol formyl chloride
A technique for pyrazole carboxyl chloride and its synthesis method, which is applied in the field of chemical synthesis of 1-methyl-3-ethyl-4-chloro-5-pyrazole carboxyl chloride, and can solve potential safety hazards in the use process and difficulties in storage and transportation , safety hazards and other issues, to achieve the effect of no three wastes, low production cost, simple and safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Add 19mmol of 1-methyl-3-ethyl-4-nitro-5-pyrazolecarboxylic acid, 0.019mmol of pyridine and 20mL of benzene into a 100mL three-necked flask, and dropwise add 10mL of 5.7mmol bis(tris Chloromethyl ester) benzene solution of carbonate, dropwise, keep warm for 20h, after the reaction is over, concentrate under reduced pressure to recover the solvent, and finally collect the fraction at 120~123°C / 1333Pa to obtain a purity of 97% and a yield of 93%. 1-Methyl-3-ethyl-4-chloro-5-pyrazolecarbonyl chloride Light yellow transparent liquid. Boiling point: 297°C / 760mmHg.
Embodiment 2
[0017] Add 19mmol of 1-methyl-3-ethyl-4-nitro-5-pyrazolecarboxylic acid, 1.9mmol of triethylamine and 30mL of toluene into a 100mL three-necked flask, and dropwise add 10mL containing 15.2mmol of bis The toluene solution of (trichloromethyl)carbonate, dropwise, heat preservation reaction for 8h, the reaction is completed, the solvent is concentrated and recovered under reduced pressure, and finally the fraction at 120~123°C / 1333Pa is collected to obtain a purity of 98% and a yield of 92 % of 1-methyl-3-ethyl-4-chloro-5-pyrazolecarbonyl chloride light yellow transparent liquid.
Embodiment 3
[0019] Add 19mmol of 1-methyl-3-ethyl-4-nitro-5-pyrazolecarboxylic acid, 0.76mmol of N-methylpyrrole and 40mL of dichloromethane into a 100mL three-necked flask, and drop 10mL of Containing 9.5mmol of bis(trichloromethyl)carbonate in toluene solution, after dripping, heat preservation reaction for 16h, after the reaction was completed, concentrated under reduced pressure to recover the solvent, and finally collected the fraction at 120~123°C / 1333Pa to obtain a purity of 97%. The yield was 94% of 1-methyl-3-ethyl-4-chloro-5-pyrazolecarbonyl chloride as light yellow transparent liquid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com