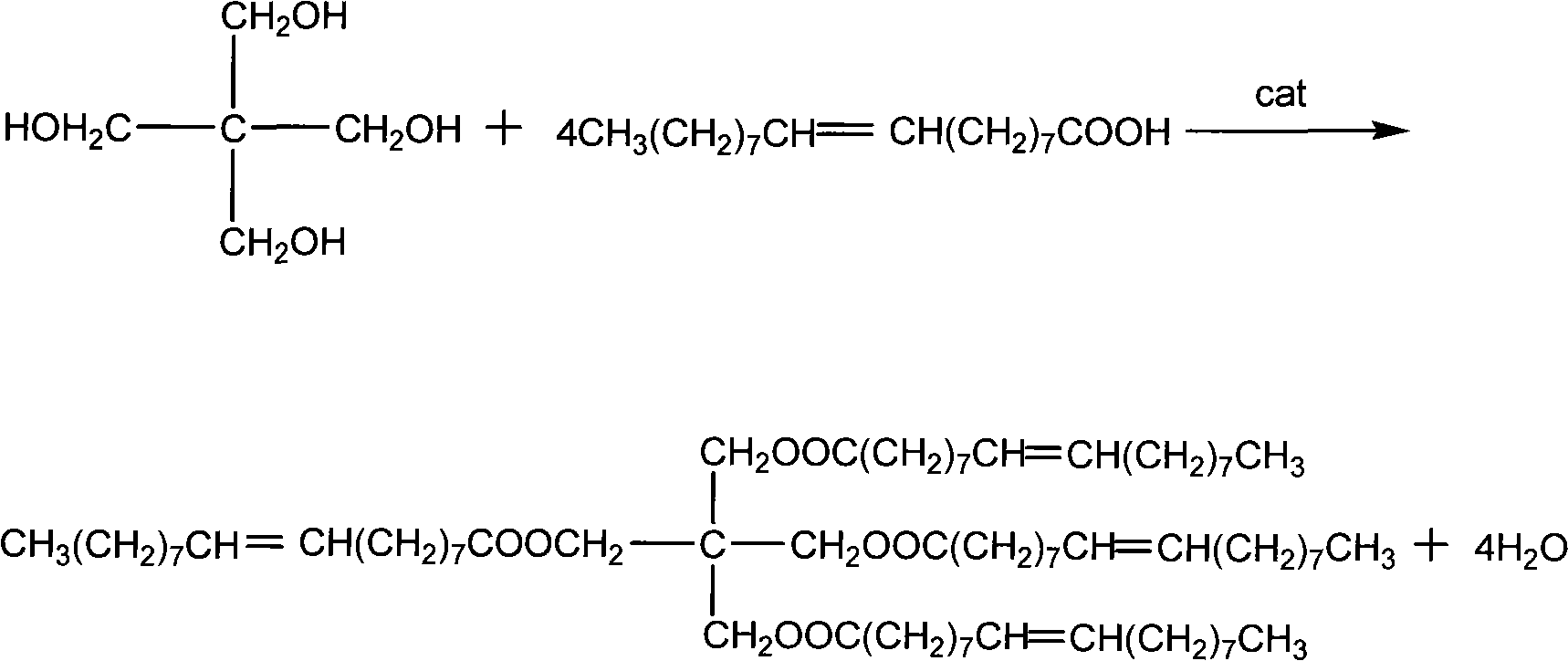Process for producing pentaerythritol oleate
A technology of pentaerythritol oleate and pentaerythritol, which is applied in the field of preparation of pentaerythritol oleate, can solve the problems of poor catalyst reusability, affecting product appearance and performance, reducing yield and product purity, etc. Raw material cost, product color improvement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] According to the preparation method of the pentaerythritol oleate of the present embodiment specifically comprises the steps:
[0021] (1). Pentaerythritol and excess oleic acid react under the catalysis of lanthanum methanesulfonate to obtain a light yellow liquid comprising product pentaerythritol oleate and excess raw material oleic acid:
[0022] Add 635.6 parts of oleic acid, 68.1 parts of pentaerythritol, 56.3 parts of toluene and 2.1 parts of lanthanum methanesulfonate into the esterification kettle, stir and heat the material to reflux under the protection of nitrogen, keep the temperature at 120-160°C, and after reflux for about 3 hours, Take a sample to measure the acid value. The reaction was stopped when the acid value was no longer significantly lowered. The catalyst is separated by filtration, and the filtered catalyst can be reused.
[0023] Wherein, the esterification reaction equation is:
[0024]
[0025] (2). The described light yellow liquid ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com