Method for solid phase polypeptide synthesis of hexarelin
A technology of solid-phase polypeptide synthesis and hexarelin, which is applied in the field of solid-phase polypeptide synthesis of hexarelin, can solve the problems of using highly toxic substances and long synthesis cycle, and achieve the reduction of three wastes, low production cost and high quality stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] (1) Preparation of Fmoc-Lys(Boc)-Linker AM Resin
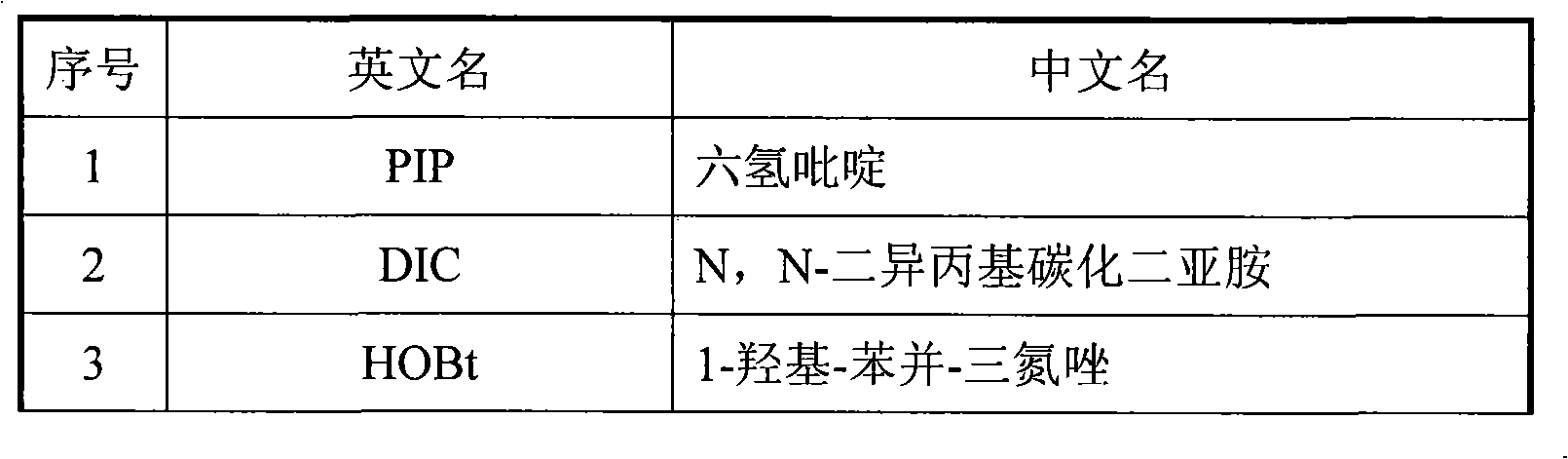
[0051] Weigh 1g of Fmoc-Linker-AM Resin (Loadding: 0.6mmol / g) into a 30ml peptide bottle, add about 15ml of DCM to the resin, soak for 10 minutes (at room temperature) and dry it with a vacuum pump, add 10ml of decapping reagent and put it in a constant temperature oscillator React for 5 minutes, dry with a vacuum pump, re-add 10ml of capping agent and react for 15 minutes (30°C-40°C), dry with a vacuum pump, wash with industrial-grade DMF for 3 times, wash with anhydrous methanol for 3 times, and re-evaporate Wash with DCM for 3 times, add Fmoc-Lys(BOC)-OH0.702g, HOBt0.337g, analytical grade DMF8ml, DIC0.2ml, Collidine0.3ml to react for 40 minutes (30℃~50℃), use vacuum pump to dry, Industrial-grade DMF was washed 3 times, anhydrous methanol was washed 3 times, redestilled DCM was washed 3 times, and dried to obtain Fmoc-Lys(Boc)-LinkerAM Resin;
[0052] (2) Preparation of Fmoc-D-Phe-Lys(Boc)-Linker AM Resin
[0053] ...
Embodiment 2
[0072] Adopt the same method and processing condition as embodiment 1, wherein:
[0073] Use Fmoc-Linker-MBHA Resin as the starting material, add decapping reagent and react at 30-50°C for 5 minutes, drain, add decapping reagent again and react for 15 minutes, wash, add Fmoc-amino acid, analytical grade DMF, Collidine, DIC / HOBt or DIC / HOAt or BOP / HOBt or BOP / HOAt or HBTU / HOBt or HBTU / HOAt or HATU / HOBt or HATU / HOAt or HCTU / HOBt or HUTC / HOAt or TBTU / HOBt, reaction 40 minutes, with embodiment 1 Operate until the hexapeptide is finished. After cutting and other reactions, 0.540 g of white powder product was obtained.
Embodiment 3
[0075] Weigh 50g of Fmoc-Linker-AM Resin or Fmoc-Linker-MBHA Resin (Loadding: 0.7mmol / g) into a 600ml peptide bottle, add decapping reagent at 30-50°C for 5 minutes, drain, add decapping reagent again 15 minutes, wash, add Fmoc-amino acid, analytical grade DMF, Collidine, DIC / HOBt or DIC / HOAt or BOP / HOBt or BOP / HOAt or HBTU / HOBt or HBTU / HOAt or HATU / HOBt or HATU / HOAt or HCTU / HOBt or TBTU / HOBt, reacted for 40 minutes, and operated with embodiment 1 until the hexapeptide was connected. After cutting and other reactions, 33g of white powder product was obtained. The purity of the crude product reacted with HBTU / HOBt will be better.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com