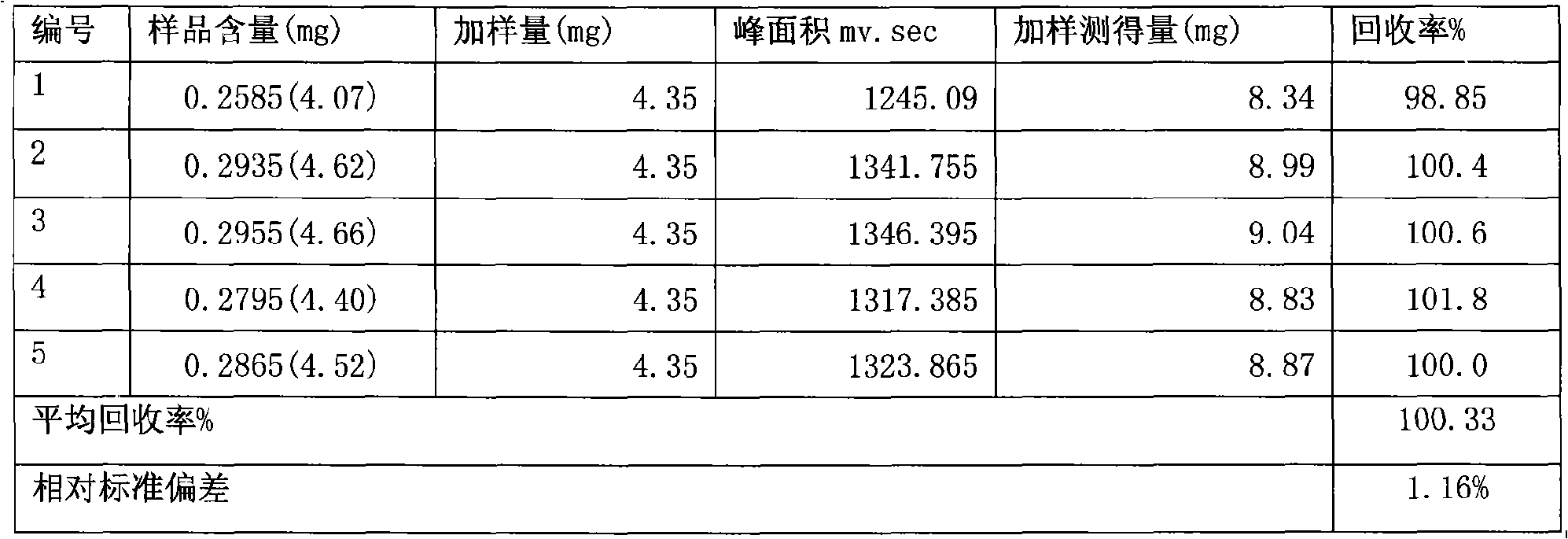
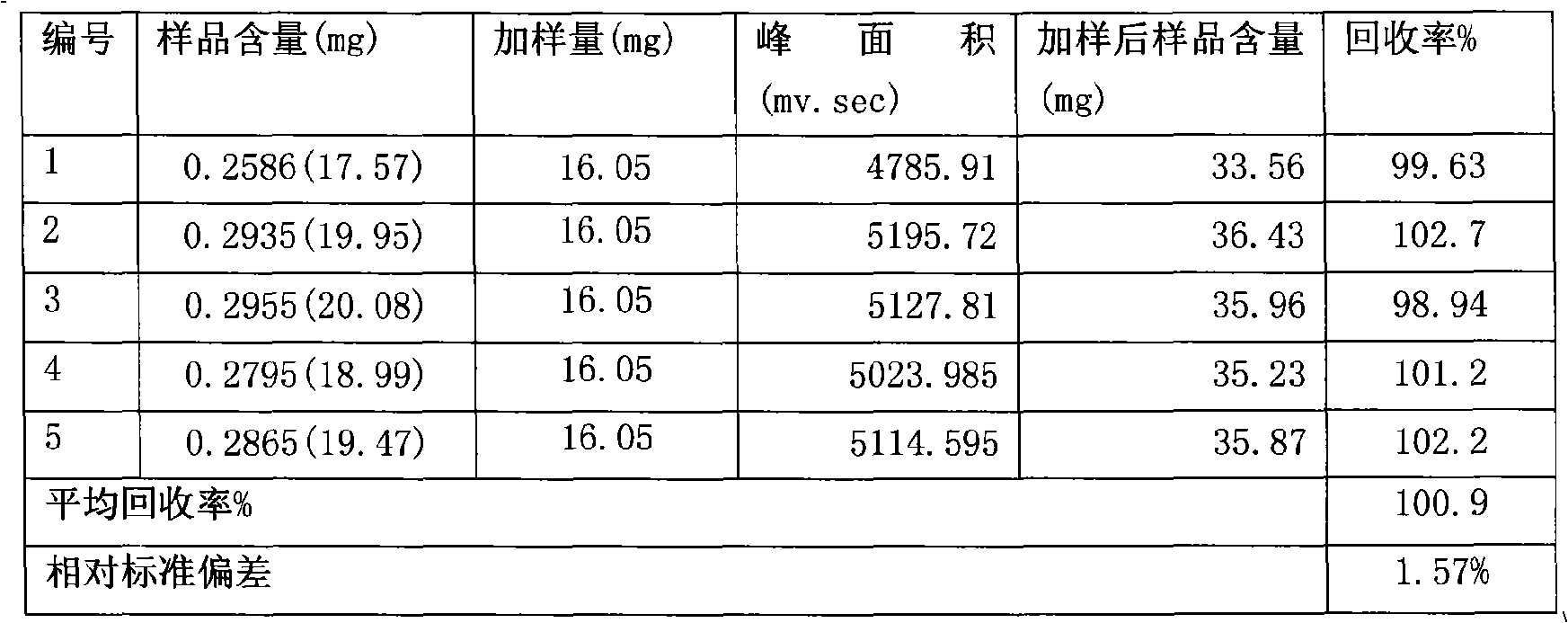
Senna formulation and quality control method
A quality control method and technology of senna, applied in the directions of pill delivery, pharmaceutical formulation, measuring device, etc., can solve the problems of low extraction rate, thermal instability of active ingredients, long heating time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1 Granules
[0020] 【Prescription】Senna Leaf 2000g
[0021] [Preparation method] Get senna leaf coarse powder (20 mesh powder), according to the percolation method under the liquid extract and extract item (Chinese Pharmacopoeia 2005 edition one appendix IO), make solvent ultrasonic with 30% ethanol three times amount After 30 minutes of treatment, carry out percolation, collect 8 times the amount of percolation liquid of medicinal materials, concentrate under low temperature and reduced pressure to a clear paste with a relative density of 1.24-1.28 (60°C) as a binder. According to the amount of total sennosides contained in the clear paste, add appropriate amount of dextrin and sucrose powder, boil and granulate, dry, pack, and make the total sennosides contained in granules of this product. , should be 40-50 mg, and made into 1000 bags of granules with a specification of 10 g per bag.
Embodiment 2
[0022] Example 2 tablet
[0023] 【Prescription】Senna Leaf 1000g
[0024] [Preparation method] Get senna leaf coarse powder (20 mesh powder), according to the percolation method under the liquid extract and extract item (Chinese Pharmacopoeia 2005 edition one appendix IO), make solvent ultrasonic with 30% ethanol three times amount After 30 minutes of treatment, carry out percolation, collect 8 times the percolation liquid of the medicinal material, concentrate under low temperature and reduced pressure to a ointment with a relative density of 1.16-1.22 (60°C), spray dry (inlet air temperature 160-170°C, outlet air temperature 50~60℃) into extract powder. According to the amount of total sennosides contained in the clear ointment measured, add an appropriate amount of starch, add an appropriate amount of silicon dioxide, and press the powder into tablets to make the tablet of this product. Each tablet contains total sennosides in terms of sennoside B, which should be 20-25 mg...
Embodiment 3
[0025] Embodiment 3 capsules
[0026] [Prescription] Senna 1000g
[0027] [Preparation method] Get senna leaf coarse powder (20 mesh powder), according to the percolation method under the liquid extract and extract item (Chinese Pharmacopoeia 2005 edition one appendix IO), make solvent ultrasonic with 30% ethanol three times amount After 30 minutes of treatment, carry out percolation, collect 8 times the percolation liquid of the medicinal material, concentrate under low temperature and reduced pressure to a ointment with a relative density of 1.16-1.22 (60°C), spray dry (inlet air temperature 160-170°C, outlet air temperature 50~60℃) into extract powder. According to the amount of total sennosides contained in the clear ointment, add appropriate amount of starch, granulate, dry, add appropriate amount of silicon dioxide, and fill capsules to make capsules of this product. Each capsule contains total sennosides and sennoside B Calculated, should be 20 ~ 25mg, made into 1000 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com