Hypoglycemic polypeptide fused protein, structure and use of derivate thereof
A fusion protein and hypoglycemic polypeptide technology, applied in the field of bioengineering, can solve the problems of short half-life of the original polypeptide, poor patient compliance, and short action time, and achieve the effects of high bioavailability, low immunogenicity, and improved stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of hypoglycemic polypeptide 1 fusion protein
[0033] Step 1: According to the amino acid sequence of hypoglycemic polypeptide 1, its gene sequence was synthesized with reference to the codon preference of Escherichia coli, and the DDDDK sequence was designed upstream of the gene, and the recognition sequences of Kpn1 and Hind3 were added at both ends. The amino acid sequence of the gene fused with TrxA is as follows: TrxA-HisTag-DDDDK-HGEGT FTSDV SSYLE GQAAK EFIAW LVKGR PSSGA PPPS Step 2: Digest the synthesized gene and pET32a vector with Kpn1 and Hind3 respectively. The products were ligated at 16°C for 8 hours, and the ligated products were transformed into E. coli competent cells according to the standard method given in "Molecular Cloning" and positive clones were selected. Sequencing verifies the accuracy of the inserted gene.
[0034] Step 3: Cultivate positive clones at 37 degrees overnight in LB medium to make seed solution, and add 50 μg / ml ampici...
Embodiment 2
[0038] Preparation of hypoglycemic polypeptide 2 fusion protein, single-chain PEGylated fusion protein derivatives, and enzymatic digestion products of derivatives and determination of hypoglycemic activity.
[0039] According to the amino acid sequence of hypoglycemic polypeptide 2, its gene sequence was synthesized with reference to the codon preference of Escherichia coli, the DDDDK sequence was designed upstream of the gene, and the recognition sequences of Kpn1 and Hind3 were added at both ends. The amino acid sequence of the gene fused with TrxA is as follows: TrxA-HisTag-DDDDK HGEGT FTSDV SSYLE GQAAK EFIAW LVKGRC
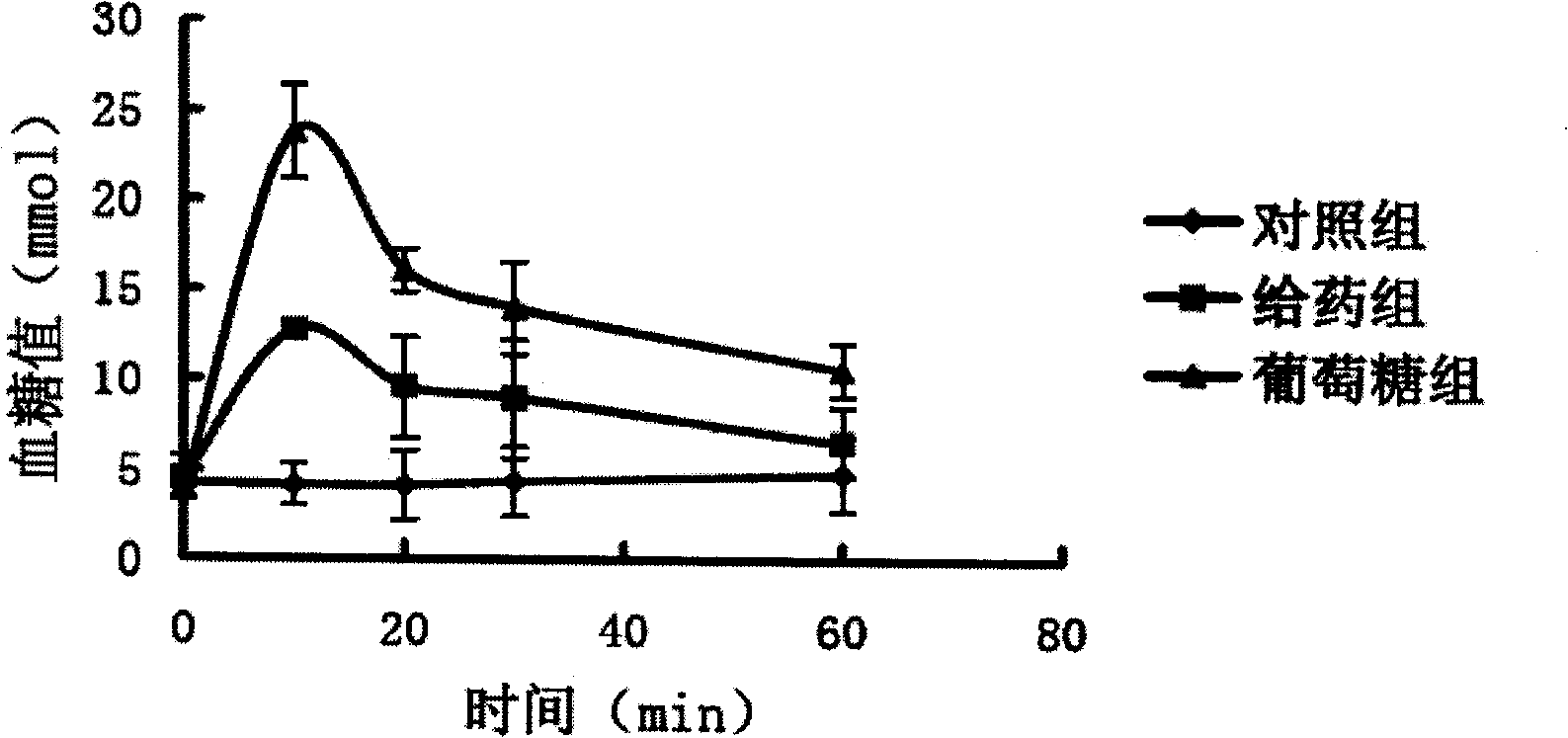
[0040] The recombinant construction, transformation, expression and purification of the gene of the TrxA-GLP-1 (Gly8, Cys37) fusion protein are the same as in Example 1. SDS-PAGE detection shows that the purity of the purified fusion protein is higher than 95%. Dilute the purified protein to 1 mg / ml, add maleimidated single-chain polyethylene glycol 5000 at a...
Embodiment 3
[0044] Preparation of hypoglycemic polypeptide 3 fusion protein and biotinylated fusion protein derivatives and determination of hypoglycemic activity.
[0045] Step 1: According to the amino acid sequence of hypoglycemic polypeptide 3, its gene sequence was synthesized with reference to the codon preference of Escherichia coli, an Arg codon was designed upstream of the gene, and recognition sequences of BamH1 and Hind3 were added at both ends. The amino acid sequence of the fusion protein composed of the gene and HisTag is as follows:
[0046] GSSHHHHHHSSGLVPRGSHMASMTGGQQMGRGSRHGEGTFTSDVSSYLEGQAAQEFIAWLVKGR
[0047] Step 2: Double digest the synthesized gene and the pET28a vector with BamH1 and Hind3 respectively, and connect the products after digestion at 16°C for 8 hours, and transform the ligated products into Escherichia coli to sense cells and select positive clones. Sequencing verifies the accuracy of the inserted gene.
[0048] Step 3: Cultivate positive clones at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com