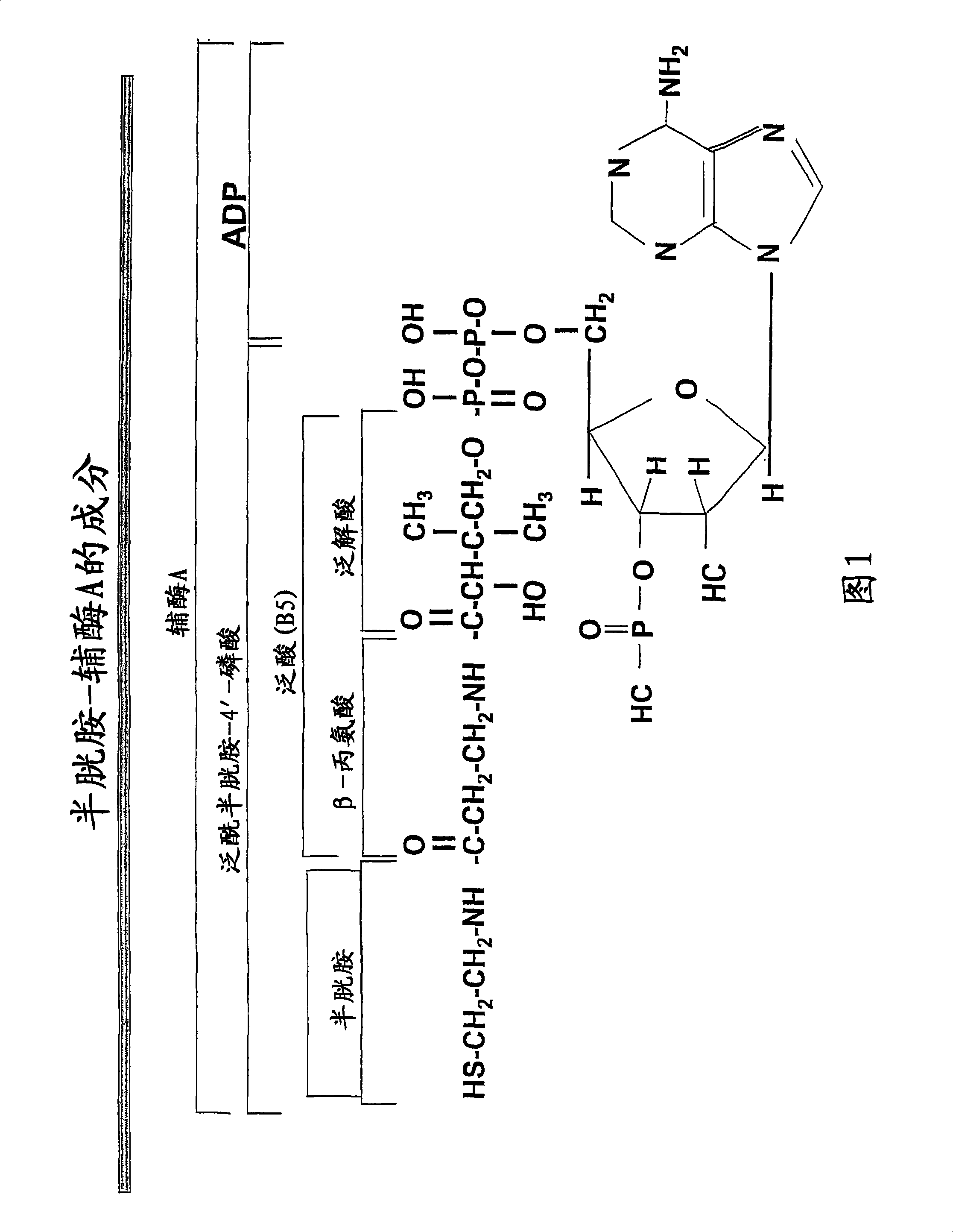
Materials and methods for treating viral infections with a cysteamine compound
A virus infection, cysteamine technology, applied in the treatment of virus-related symptoms, infecting animals with I-V viruses, preventing or delaying the occurrence of virus-related complications, can solve side effects, harmful host cell DNA replication, decreased therapeutic efficacy, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1 - Treatment of Influenza-Related Symptoms
[0070] A man with influenza virus infection who presented with symptoms associated with influenza infection (runny nose, fever, tiredness) was initially treated with over-the-counter nasal decongestants and mucolytics. Within 24 hours, the over-the-counter drug is not effective in treating flu-related symptoms.
[0071] After over-the-counter medications proved ineffective, the subject was orally administered cysteamine hydrochloride at a dose of approximately 700 mg. Within 24 hours, symptoms associated with influenza infection had disappeared. The subject displays a general sense of well-being.
Embodiment 2
[0072] Example 2-Studies on the Antiviral Activity of Cysteamine on H5N1 Avian Influenza Virus: Using Phosphoric Acid In vitro and in vivo studies of oseltamivir as a control
[0073] According to one embodiment of the present invention, cysteamine exhibits antiviral activity against H5N1 avian influenza virus. The subject-matter of the invention is particularly advantageous due to the unexpected results with avian influenza viruses. For example, as described below, cysteamine was particularly effective in the treatment of H5N1 avian influenza virus, even more effective than the registered anti-avian influenza drug oseltamivir phosphate (whose generic name is Tamiflu (TAMIFLU )) Much better.
[0074] Materials and methods
[0075] Cysteamine (hereinafter referred to as "TG21", containing 99% cysteamine) was provided by Omega Bio-Pharma (H.K.) Limited. Embryo eggs from specific pathogen-free (SPF) hens (Beijing, China) were used in this experiment. The CV strain of...
Embodiment 3
[0105] Antiviral activity of embodiment 3-cysteamine against H5N1 type avian influenza virus in mice Materials and methods
[0106] Cysteamine (hereinafter referred to as "TG21", containing 99% cysteamine) was provided by Omega Bio-Pharma (H.K.) Limited. H5N1 avian influenza virus WV strain was isolated from infected chickens. As described here, using Tamiflu (Shanghai, China, Roche (China) Co., Ltd.).
[0107] Evaluation of the 50% lethal dose (mLD50) of H5N1 avian influenza virus in mice
[0108] The preservation solution of H5N1 avian influenza (WV strain) was firstly diluted 1:5 with PBS, and then serially diluted by four times to obtain 5 dilutions (1:5 to 1:1280). Six- to eight-week-old female mice were anesthetized by intramuscular injection of 100 μL of 1% barbital sodium, and then the mice were inoculated by dripping 50 μL of diluted H5N1 avian influenza virus WV strain into the nasal cavity of each mouse (n=each diluted 10 mice). Animals were monitored da...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com