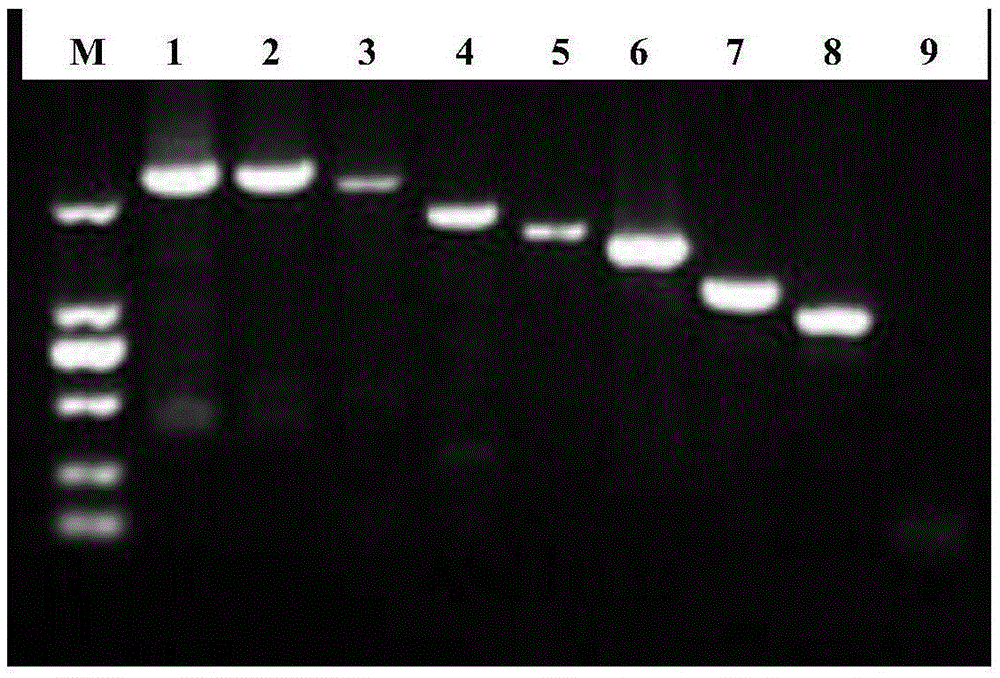
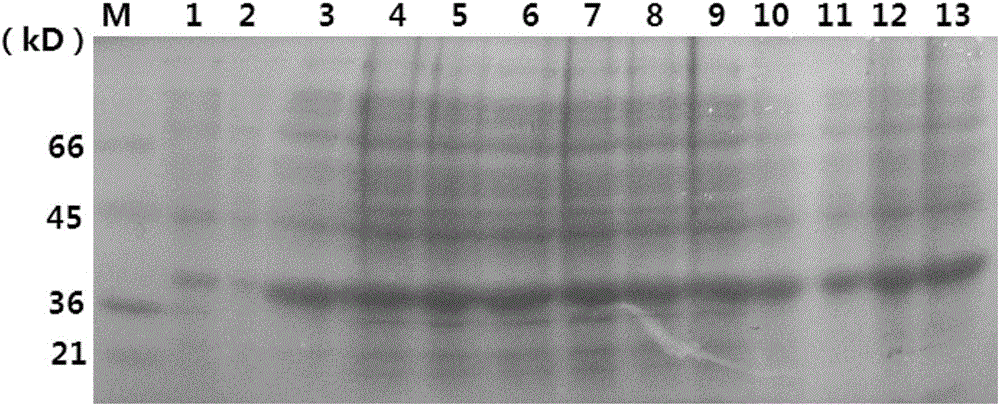
Patents
Literature
79 results about "Avian bornavirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In 2008, by pyrosequencing of cDNA from the brains of several parrots suffering from proventricular dilatation disease (PDD), Honkavuori et al. identified the presence of a novel bornavirus.
Avian influenza virus live attenuated vaccine and uses thereof
ActiveUS20110150912A1Poor replicationLess virulentSsRNA viruses negative-senseBacteriaHeterologousDonor strain
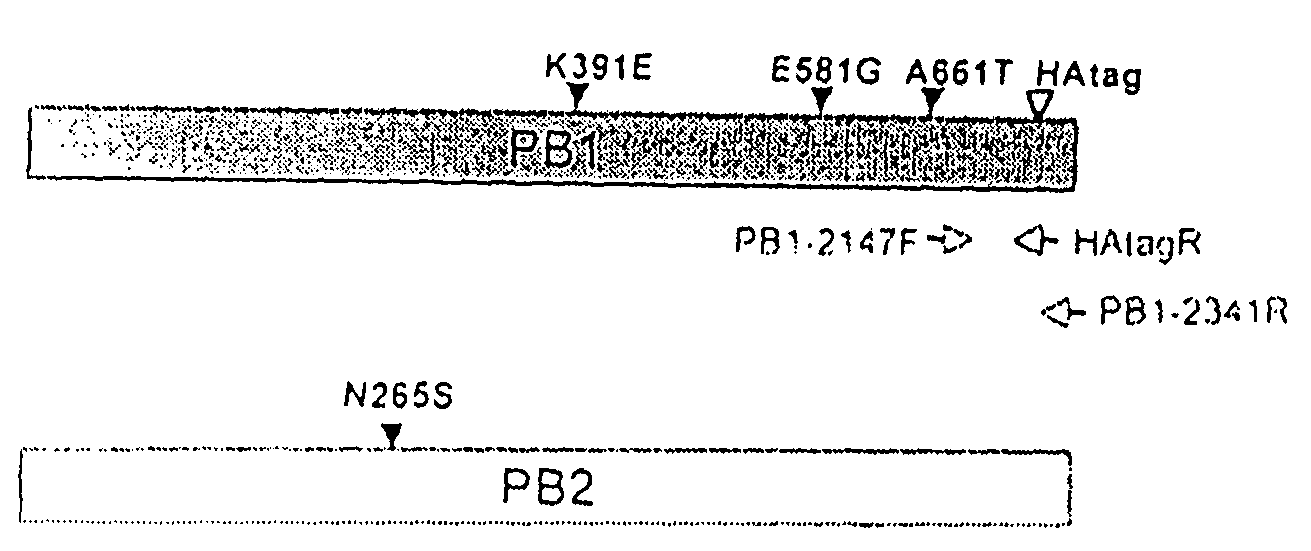
Described in this application are attenuated strains of avian influenza virus containing temperature sensitive mutations in addition to a genetic tag in the PB1 gene. The attenuated viruses are useful as avian and mammalian vaccine for protective immunity against homologous and heterologous lethal challenges with influenza virus. A genetically modified avian influenza virus backbone is described which can be used as a master donor strain for the generation of live attenuated vaccines for epidemic and pandemic influenza.
Owner:UNIV OF MARYLAND
H5, H7, H9 subtype auian flu virus real-time fluo rescent quantixative PCR detecting method
InactiveCN1814805AStrong specificityGood linear relationshipMicrobiological testing/measurementFluorescenceBio engineering
This invention relates to a real time fluorescence quantitative PCR test method for H5, H7 and H9 sub-avian flu viruses including the following steps: set-up of standard molecules, the design of specific virus quantitative PCR primer and the probes, pick-up of virus sample RNA, the inverse transcription polyenzyme chain reaction to the pic-up sample RNA to get a sample cDNA, the optimization and set-up of the PCR quantitative test method for M genes.
Owner:SHANGHAI JIAO TONG UNIV
Antigenic class of avian reoviruses
InactiveUS6951650B1Effectively affords protection in poultryEffective protectionViral antigen ingredientsDigestive systemFowlDisease
The present invention provides a new antigenic class of avian reoviruses which are involved in enteric disease conditions in poultry. Moreover, the invention provides a vaccine for use in the protection of poultry against such disease conditions derived from the new type of avian reoviruses.
Owner:INTERVET INT BV
Composite kit for detecting avian influenzas and Newcastle disease viruses and detection method
InactiveCN101798602AQuality improvementEasy to operateMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceBiology
The invention relates to a composite kit for detecting avian influenzas and Newcastle disease viruses and a detection method, characterized in that the kit comprises a lysis solution, an RT-PCR buffer solution, primers and a probe mixture solution, particularly a primer and a probe sequenc for detecting various subtype avian influenzas viruses, a primer and a probe sequenc for detecting Newcastle medium and strong viruses, enzyme mixture, DEPC water and negative and positive contrasts. The detection method comprises the following steps of: carrying out an RT-PCR reaction and judging a quality control standard and results. The invention has the advantages of high sensitivity, good specificity and simple operation. The process from processing samples to getting a result only needs 4 hours. The invention overcomes the defects of single fluorescence and realizes the aim of simultaneously detecting two viruses by one real-time fluorescent quantification PCR (Polymerase Chain Reaction) reaction.
Owner:INSPECTION & QUARANTINE TECH CENT SHANDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
New castle disease and H9 subtype bird flu bivalent vaccine
ActiveCN104922663AImproving immunogenicitySmall dose of immunizationViral antigen ingredientsAntiviralsDiseaseOil adjuvant
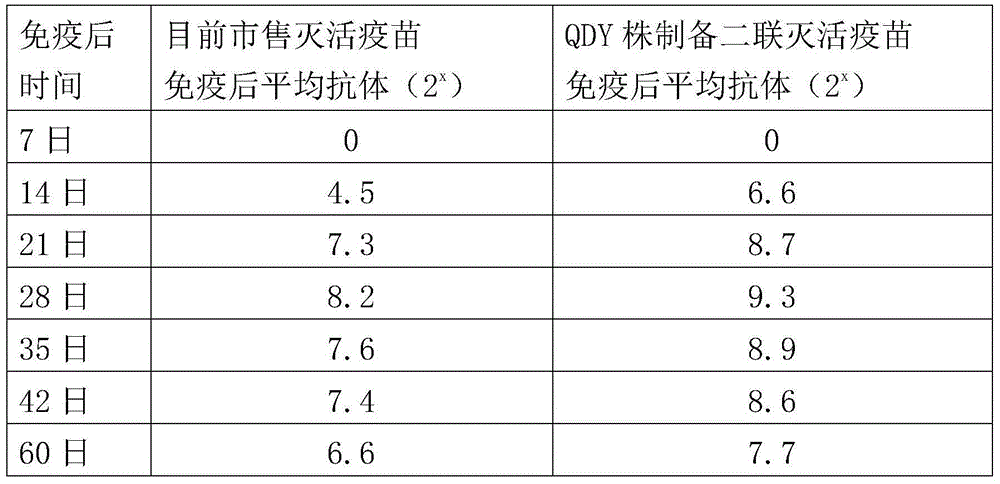
The invention aims at providing a new castle disease and H9 subtype bird flu bivalent vaccine. The new castle disease and H9 subtype bird flu bivalent vaccine contains antigens and adjuvant. The antigens are inactivated H9 subtype bird flu viruses and new castle disease viruses. The H9 subtype bird flu viruses are QDY strains, and the preservation number of the H9 subtype bird flu viruses is CCTCC v201517. The QDY strains of the H9 subtype bird flu viruses and a Lasota strain of the new castle disease viruses are inoculated to chick embryos respectively, and then virus liquid is collected; the virus liquid and the oil adjuvant are mixed and emulsified into the vaccine after the virus liquid is inactivated through a formaldehyde solution. The new castle disease and bird flu bivalent inactivated vaccine is good in immunogenicity, antibody production is fast after immunity, the produced antibody titer is high, the produced antibody holding time is long, the retention period is long, the immunizing dose is small, the selected adjuvant is easy to inject, and two kinds of diseases can be prevented through one-time injection. The vaccine has the advantages of being efficient and good in safety.
Owner:YEBIO BIOENG OF QINGDAO
Nucleic acid detection kit for synchronously identifying and diagnosing newcastle disease virus and avian influenza virus
InactiveCN101240352AAvoid potential risksEasy to operateMicrobiological testing/measurementHighly pathogenicFluorescence
The invention belongs to the field of inspection and quarantine technology. Specifically, the invention is a nucleic acid detection reagent kit for synchronous discriminating and diagnosing avian influenza virus and newcastle disease virus. The detection reagents of the reagent kit include extraction reagent for extracting virus by silicon gel absorption column method, detection amplification reagent for detecting nucleic acid by RT-PCR Taq Man fluorescent probe method, and pretreatment liquid for solid tissue specimen for extracting virus RNA. Further, the invention employs in vitro transcription RNA as a positive contrast of the reagent kit. The reagent kit can rapidly and synchronously discriminate and diagnose avian influenza virus and newcastle disease virus which are highly infectious among avian plagues and have similar symptom, determine current major prevalent subtypes, such as H5, H7, H9, etc., and discriminate whether a infection source is an avian influenza having high pathogenicity, non-pathogenic avian influenza or mildly pathogenic avian influenza to human. The reagent kit is suitable for livestock and veterinarian station, import and export inspection and quarantine bureau, as well as other laboratories, and can be used for large-scale detection of influenza and epidemic surveillance.
Owner:SHANGHAI KEHUA BIO ENG
Avian influenza virus live attenuated vaccine and uses thereof
Described in this application are attenuated strains of avian influenza virus containing temperature sensitive mutations in addition to a genetic tag in the PB1 gene. The attenuated viruses are useful as avian and mammalian vaccine for protective immunity against homologous and heterologous lethal challenges with influenza virus. A genetically modified avian influenza virus backbone is described which can be used as a master donor strain for the generation of live attenuated vaccines for epidemic and pandemic influenza.
Owner:UNIV OF MARYLAND
Establishment of MDCK cell line stably expressing beta-galactoside alpha-2, 3-sialytransferase I(ST3GAL I)
The invention provides an MDCK-ST3GAL I cell line, concretely an MDCK cell line stably expressing beta-galactoside alpha-2, 3-sialytransferase I(ST3GAL I) with the preservation number of CGMCC No. 2620; the invention further provides a method for establishing the MDCK-ST3GAL I cell line and a method for improving the growth titre of avian influenza virus and the forming capability of plaque. The invention further provides an application of the MDCK-ST3GAL I cell line in researching avian influenza virus 2, 3 linked receptor tendency; and the invention is used for applications in monitoring the avian influenza virus receptor associative property variant strain, screening medicines resisting avian influenza virus, measuring the neutralizing antibody and mass producing cell culture vaccine.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Method for detecting different subtype avian influenza viruses and special kit thereof
InactiveCN101724713AImprove throughputEasy to operate and save timeMicrobiological testing/measurementFluorescence/phosphorescenceAgricultural scienceHigh flux
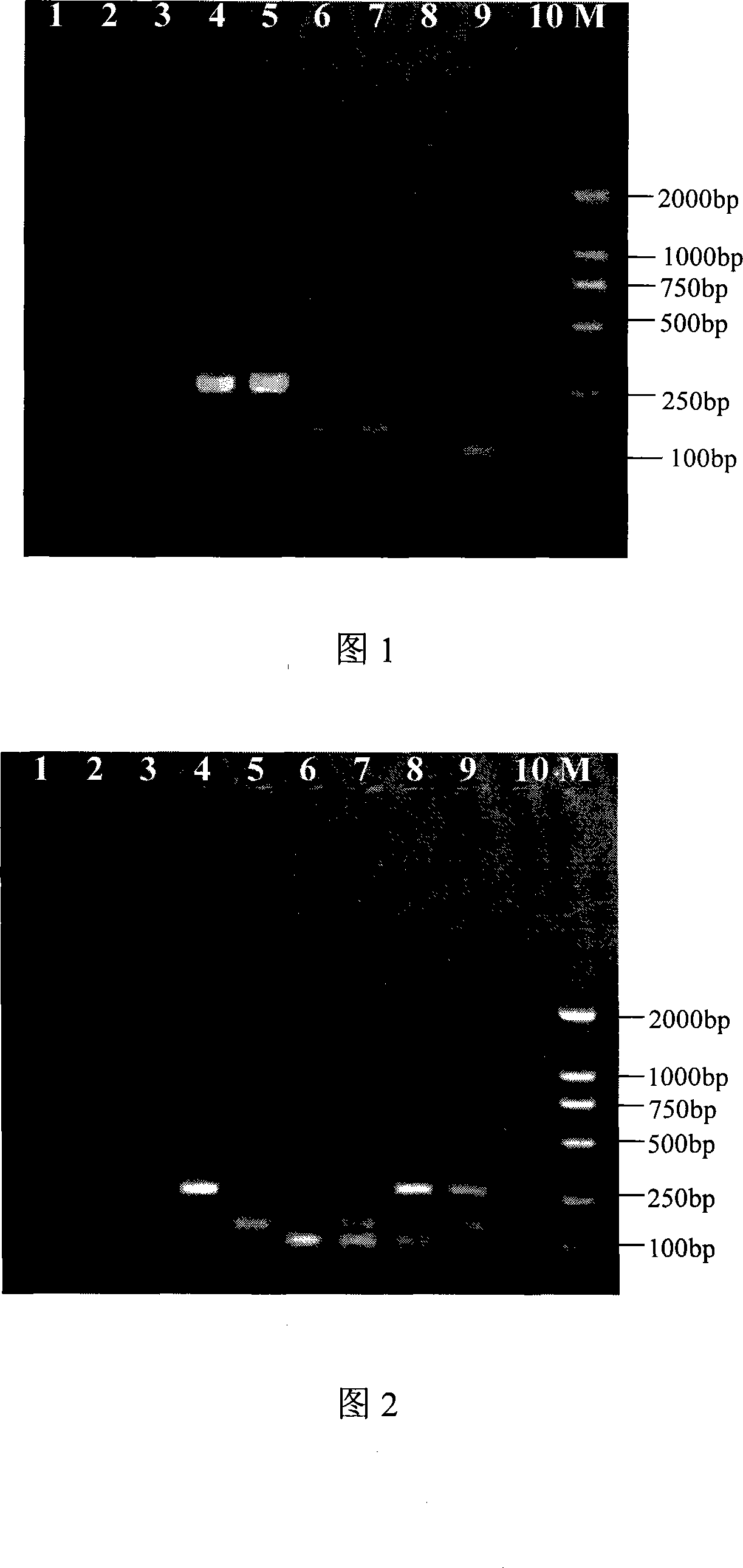
The invention discloses a method for detecting different subtype avian influenza viruses and a special kit thereof. The method comprises the following steps of:, carrying out multiple PCR using a primer pair B by a primer pair A by utilizing the cDNA of a biological sample to be tested as a template, carrying out agarose gel electrophoresis on products of the multiple PCR, and respectively carrying out gel scanning and analysis by a fluorescence imaging analyzer with the wavelength of 532 nm and 635 nm; if a fluorescence strip exists under the wavelength of 635nm, judging that the biological sample to be tested contains H3 subtype avian influenza virus; and if a fluorescence strip exists under the wavelength of 532nm, judging that the biological sample to be tested contains H6 subtype avian influenza virus. The primer pair A is a primer pair consisting of nucleotides represented by a sequence 11 and a sequence 12 in a sequence list, and the 5' end of the nucleotides represented by the sequence 12 is marked by Tamra. The primer pair B is a primer pair consisting of nucleotides represented by a sequence 3 and a sequence 4 in the sequence list, and the 5' end of the nucleotides represented by the sequence 4 is marked by Cy5. In the invention, the method has the advantages of high flux, high distinguishability, strong maneuverability, wide application range, time saving, test consumable saving, safety and the like.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Gene chip for detection and typing of bird flu virus
The present invention relates to gene chip for the detection and subtyping of bird flu virus. The present invention establishes gene chip with high specificity, high sensitivity and great practicability for detection and subtyping of bird flu virus through designing and screening 50 pairs of specific primers, 2 universal primers, 52 specific probes and 3 quality control probes for the multiple RT-PCR subtyping detection of bird flu virus genes based on the gene sequences of different bird flu virus subtypes Genebank reports; and performing the effectiveness test, specificity test, sensitivity test and condition optimization of gene chip detection. The present invention establishes also the detection and subtyping process with the chip and gives the initial application.
Owner:中国检验检疫科学研究院动植物检疫研究所
Preparation method and applications of H5N1 subtype bird flu pseudovirion
InactiveCN102191223ANo diseaseWill not cause diseaseMicrobiological testing/measurementMicroorganism based processesBird fluBiology
The invention relates to a preparation method and applications of an H5N1 subtype bird flu pseudovirion. Specifically, the application relates to a bird flu pseudovirion with packaging plasmid and the HA gene and NA gene of the highly virulent form of the bird flu virus and a preparation method and applications thereof; and the application also relates to a system for preparing the pseudovirion.
Owner:SHANGHAI INST OF BIOLOGICAL SCI CHINESE ACAD OF SCI
Primer system and method for amplifying avian influenza virus whole genomes
The invention provides a primer system and a method for amplifying avian influenza virus whole genomes. Specifically, the primer system comprises a primer pair, namely UAIV-F and UAIV-R, for amplifying the avian influenza virus whole genomes, as well as eight segmental primer pairs; and the primer pair comprises forward sequence: 5'-AGCRAAAGCAGG, and backward sequence: 3'-AGTAGAAACAAG. According to the first method, virus RNA is taken as a template, and one-step RT-PCR amplification is carried out by virtue of the UAIV-F and the UAIV-R, so as to obtain the avian influenza virus whole genomes; and according to the second method, amplification is carried out by taking virus RNA as a template so as to obtain cDNA and then amplification of eight segments of the avian influenza virus whole genomes is carried out respectively by virtue of the eight segmental primer pairs. The primer system and the method disclosed by the invention are applicable to amplification of all avian influenza virus whole genomes, including the genomes of the various subtype avian influenza viruses as well as quite conserved non-coding regions at two ends of the genomes; therefore, the invention is broad in applicability.
Owner:ZHAOQING DAHUANONG BIOLOGIC PHARMA +1
Primer pair for identifying newcastle disease virus and multi-subtype avian influenza virus and application thereof
InactiveCN102321769AStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesNewcastle disease virus NDVVirus influenza
The invention discloses a primer pair for identifying newcastle disease virus and multi-subtype avian influenza virus and an application thereof. The PCR primer pair composition provided by the invention comprises primer pair NDV, primer pair H3V, primer pair H5V, and primer pair H9V; the PCR primer pair composition is applicable to the preparation of reagents for the diagnosis or auxiliary diagnosis of newcastle disease and avian influenza, or to the preparation of reagents for the identification or auxiliary identification of newcastle disease virus and multi-subtype avian influenza virus. When the PCR primer pair composition provided by the invention is used to simultaneously detect newcastle disease virus, H3, H5 and H9-subtype avian influenza virus, the specificity is strong; the sensitivity is 1EID50 / 100 microliters, 1EID50 / 100 microliters, 1*10-2EID50 / 100 microliters, and 1EID50 / 100 microliters respectively; compared with identification results of routine test methods such as virus isolation and hemagglutination inhibition tests, the results obtained by using the PCR primer pair composition of the invention has a coincidence rate of up to 100%; the PCR primer pair composition provided by the invention is applicable to the development of corresponding multiplex RT-PCR kits for the clinical diagnosis and epidemiological control of newcastle disease and avian influenza.
Owner:CHINA AGRI UNIV
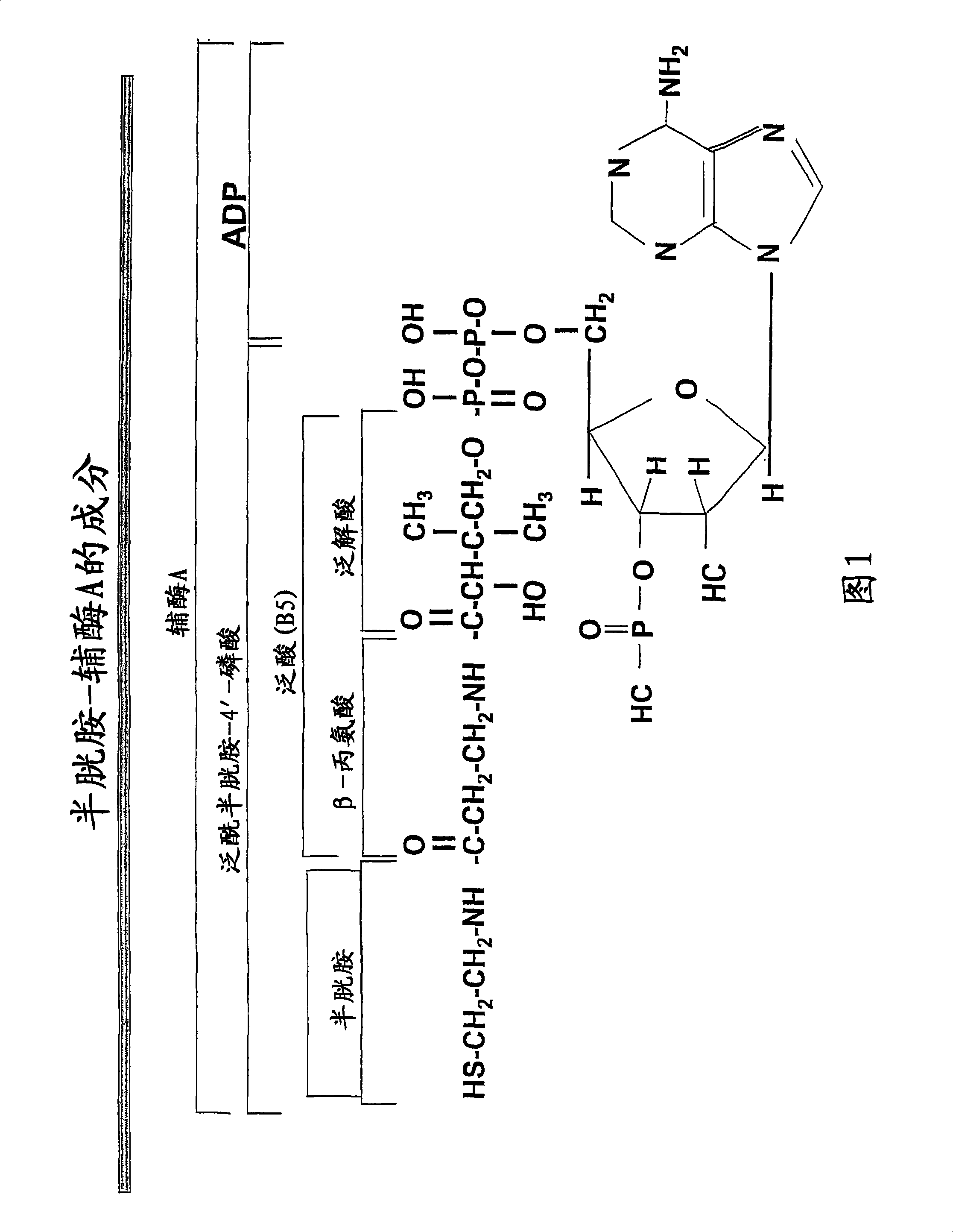
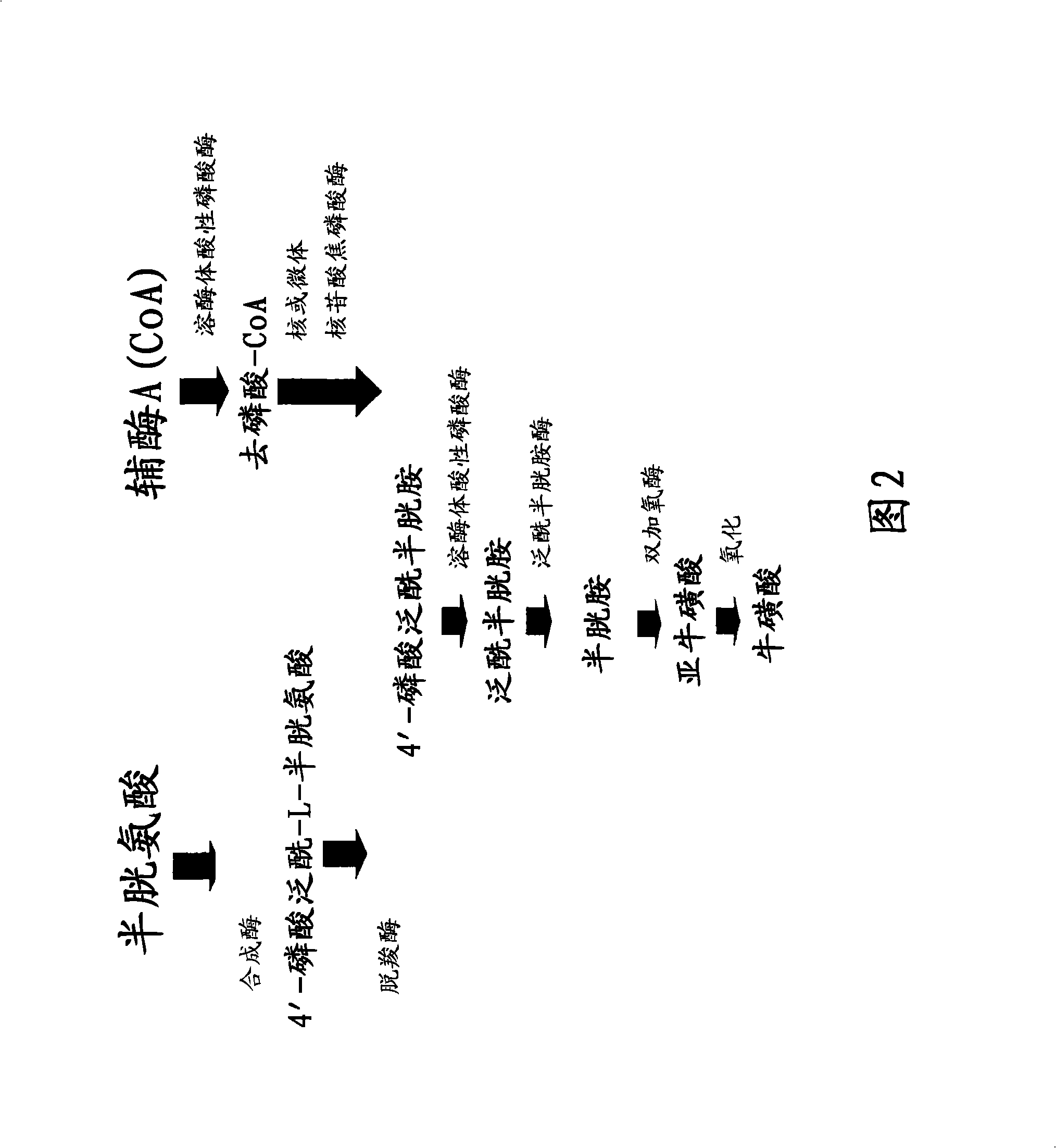
Materials and methods for treating viral infections with a cysteamine compound
The subject invention provides materials and methods for treating various health conditions, including the prevention and / or treatment of a viral infection. In a preferred embodiment, a cysteamine compound is administered to a subject to treat an influenza virus infection. More preferably, a cysteamine compound is administered to a subject to treat influenza A, influenza B, influenza C virus infections, including avian influenza virus subtypes (such as H5N1 avian influenza virus).
Owner:OMEGA BIOPHARMA H K
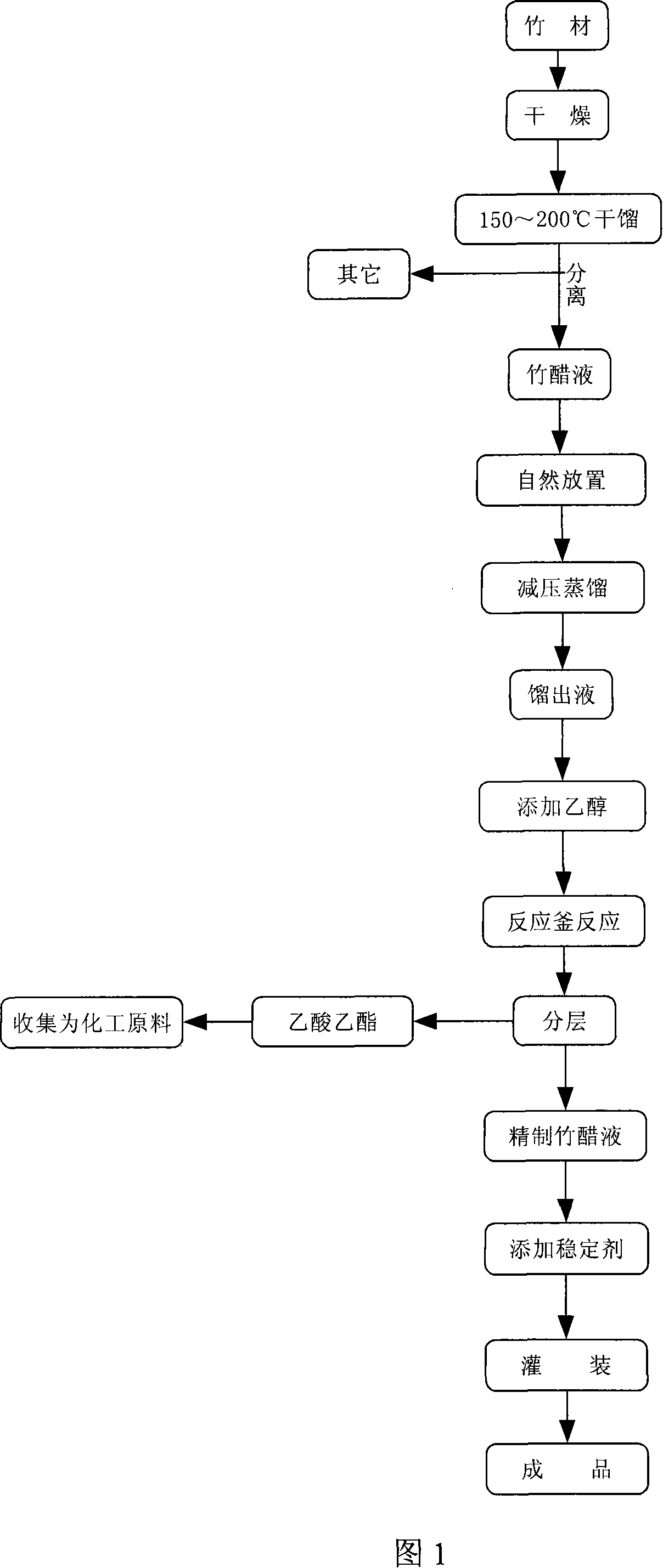
Method for preparing bamboo vinegar liquid extract for eliminating fowl influenza virus in environment
The invention relates to a bamboo vinegar extraction used for effectively removing avian and poultry influenza virus from environment such as air and soil, and for preventing avian and poultry influenza virus in eliminating environment of human and avian cross-infection and a preparation method thereof. The preparation method is divided into the steps that: the bamboo is crushed and is naturally dried or dried in other way to lead the bamboo moisture content to be 10-15 percent; then the bamboo is heated in a dry distillation reactor to raise the temperature to 150-200 DEG C for 5-7 hours to collect the bamboo vinegar liquid; the collected bamboo vinegar, after natural setting for 7-49 days, is filtrated, thereafter, the distillate liquid is collected after decompression distillation for 2-5 hours; the collected distillate liquid is added with ethanol and put into a reactor vessel for 2-4 hours under temperature of 150-180 DEG C and atmospheric pressure of 1.1-1.4; fine bamboo vinegar liquid is made by layered and removed of generated ethyl acetate. At last the final product is obtained by added with stabilizer. The invention has the advantages of convenient use, nature and environment protective and can effectively eliminate the avian and poultry influenza virus in environment.
Owner:ZHEJIANG FORESTRY ACAD
Triple fluorescence quantitative reverse transcription-polymerase chain reaction (RT-PCR) detection kit for H7N9 avian influenza virus
ActiveCN103255237AStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesConserved sequenceFluorescence
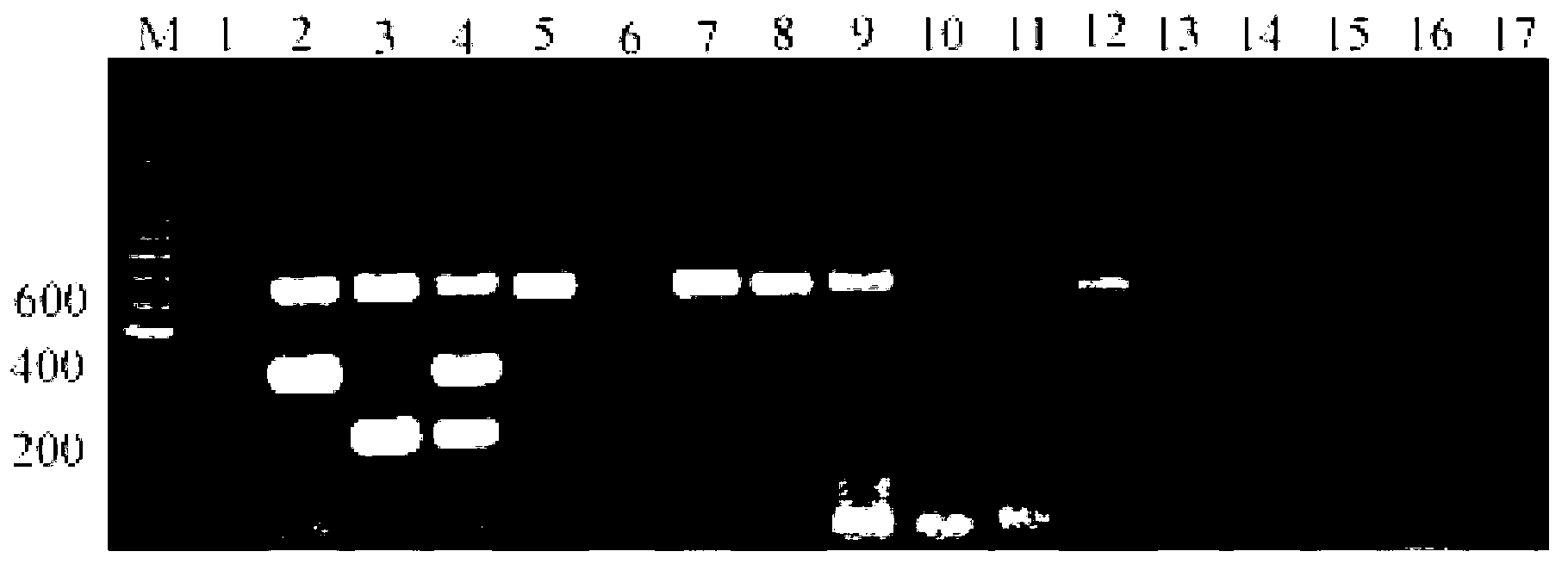
The invention discloses a triple fluorescence quantitative reverse transcription-polymerase chain reaction (RT-PCR) detection kit for an H7N9 avian influenza virus, as well as a primer pair or a primer pair group for identifying an H7 subtype avian influenza virus and an N9 avian influenza virus and generally detecting all the avian influenza viruses, and a kit thereof. Three pairs of specific primers which specially aim at the conserved sequences of an H7 subtype avian influenza virus HA gene, an N9 subtype avian influenza virus NA gene and all the avian influenza virus M genes are designed by the inventor and are combined to be the triple fluorescence quantitative RT-PCR detection kit for the H7N9 avian influenza virus, and the triple fluorescence quantitative RT-PCR detection kit can be used for determining whether H7N9 avian influenza virus infection exists or not through one-pipe detection. The primer pairs, the primer pair groups or the detection kit can be used for detecting whether samples to be detected are infected by the H7N9 subtype avian influenza virus, the H7 subtype avian influenza virus, the N9 subtype avian influenza virus and other subtype avian influenza viruses, have important significance in effectively preventing and controlling avian influenza and cutting off the transmission route of the avian influenza, and has wide application prospect.
Owner:GUANGXI VETERINARY RES INST
Method for producing newcastle disease, infectious bronchitis, egg drop syndrome and avian influenza (H9 subtype) combined inactivated vaccine
InactiveCN102038949AProduced fastHigh potencyViral antigen ingredientsAntiviralsDiseaseInfectious bronchitis
The invention relates to a method for producing a newcastle disease, infectious bronchitis, egg drop syndrome and avian influenza (H9 subtype) combined inactivated vaccine. A newcastle disease virus La Sota strain, a gallinaceous infectious bronchitis virus M41 strain, an egg drop syndrome virus NE4 strain and an A-type avian influenza virus (H9 subtype) YBF003 strain serve as production virus strains, and are mixed with a high-quality adjuvant to be prepared into the inactivated vaccine through a super concentration process; after an animal is immunized by the vaccine, an antibody is rapidly produced, the titer is high, the protection period is long, and occurrence and spread of diseases are reduced.
Owner:YEBIO BIOENG OF QINGDAO
Kit for real-time fluorescence RT-PCR detection of H7 subtype avian influenza virus
InactiveCN101736091AHigh sensitivityEfficacy monitoringMicrobiological testing/measurementSerum igeThroat swab
The invention relates to a kit for real-time fluorescence RT-PCR detection, in particular to a kit for early and rapid diagnosis of the infection of an H7 subtype avian influenza virus by using the one-step real-time fluorescence reverse transcription-polymerase chain reaction (RT-PCR) technology. Since the kit has high sensitivity and specificity, the rapid and early detection and quantitative analysis of the H7 subtype avian influenza virus in samples, such as the throat swab, the cloacae swab, the muscle or the viscera sample, the sera or the plasma and the like, can be realized by the kit.
Owner:DAAN GENE CO LTD
Test for identifying and diangnosing enzyme-linked immunosobent of natural infective minnows for avian influenza
A method of utilizing enzyme - linked immunosorbent assay to diagnose and identify naturally chicken herd of avian flu uses pronucleus to present NS 1 protein of H9N2 avian flu virus and uses it as antigen to coat reaction plate of enzyme mark. The method can identify and diagnose naturally infected chicken herd of avian flu and immunization chicken herd by using indirect enzyme - lined immunosorbent assay to determine level of antibody IgG in serum to be tested in reaction with antigen.
Owner:SHANGHAI JIAO TONG UNIV
Type H5 and H9 avian influenza virus and multiple detection method for newcastle disease virus
The invention discloses a method of detecting whether the birds and the related products are infected with H5 type or H9 type bird-flu virus or Newcastle disease virus. In the method of the invention, the trachea, cloacal swabs, blood, dung, or organs and tissues of dead birds are sterilely taken, and then milled into milk suspension by sterile physiological saline; the genomic RNA are extracted, and the RNA is used as template; the H5A, H9A and F are used as the primer for the targeted gene to undergo RT-PCR; the product obtained is electrophoresed; whether the birds detected are infected with H5, H9 type bird-flu virus or Newcastle disease virus are analyzed according to the electrophoretogram.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Nucleic acid detecting method for H5 hypotype fowl influenza virus and kit thereof
ActiveCN101016570AReduce cumbersome stepsHigh sensitivityMicrobiological testing/measurementConserved sequenceFluorescence
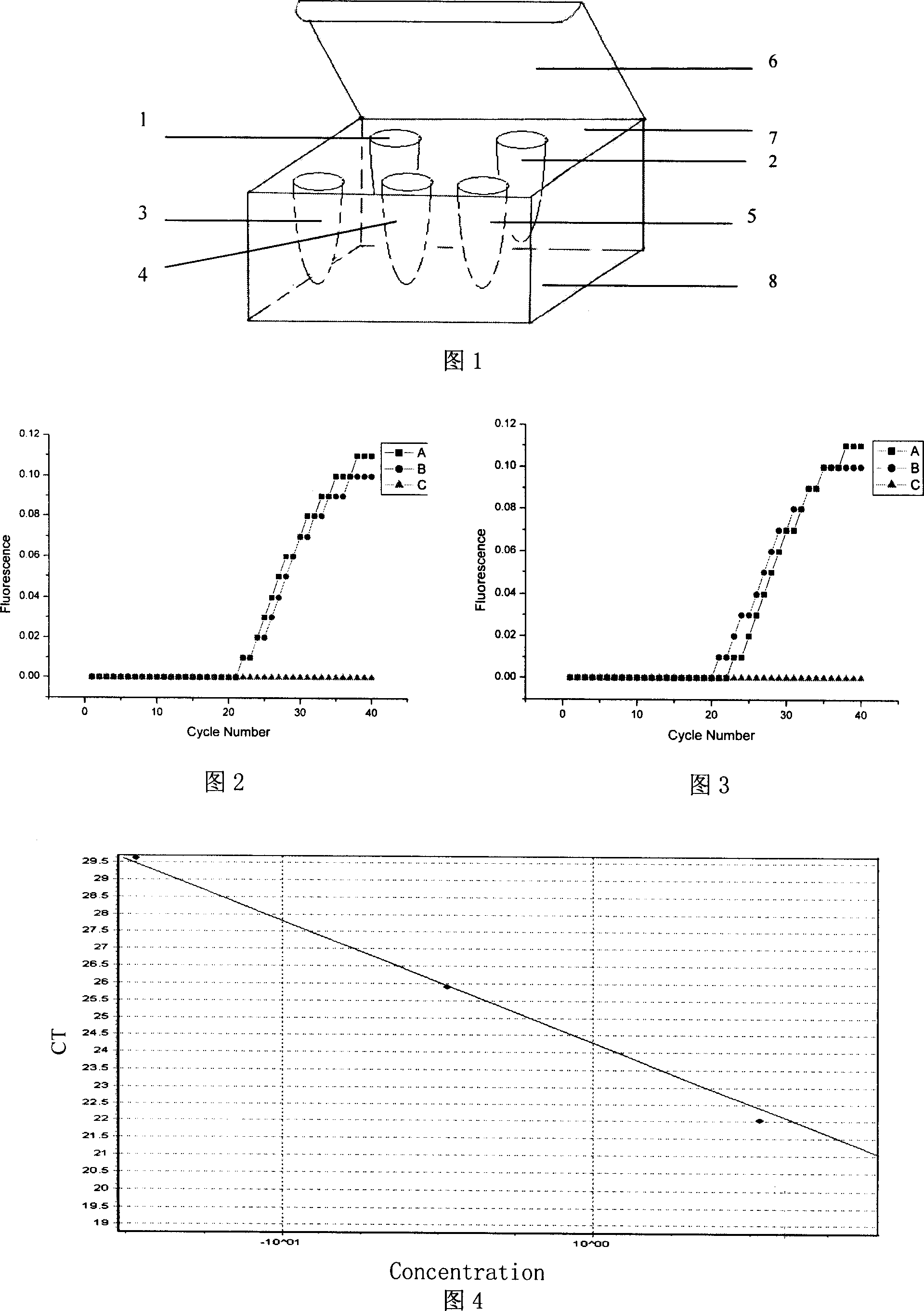
The invention discloses a detection method of H5 hypotype fowl influenza virus nucleic acid and agent case with simple operation, high reliability, high sensibility and short periodic time, which comprises the following steps: 1) finding conservative sequence of H5 hypotype fowl influenza virus as program target gene; 2) designing and synthesizing a couple of specificity primer with length at 18-30bp; 3) basing on the conservative sequence; designing and synthesizing a couple of Y type double chain specificity fluorescent probe; 4) constituting detecting solution of 20mul fluorescence PCR reaction system with the specificity primer of step 2 and Y type double chain specificity fluorescent probe and PCR composition; embedding into PCR reacting pipe; 5) setting positive for comparison mold and negative for comparison mold; proceeding actual time fluorescent PCR reaction; getting the testing result.
Owner:ZHANGZHOU BOXIN BIOLOGICAL TECH CO LTD
Photosensitizers and MRI enhancers
InactiveCN101237883AEnergy modified materialsIn-vivo testing preparationsDiabetic retinopathyProstate cancer
The present invention relates to the use of a compound of formula 3 or a salt thereof to prepare a medicament or phototherapeutic agent for treating the following diseases, including: acne; AIDS; viral hepatitis; diabetic retinopathy; SARS virus infection; coronavirus Arterial stenosis; carotid artery stenosis; intermittent claudication; Asian (chicken) avian influenza virus infection; cervical dysplasia or various cancers, including: blood cancer, cervical cancer, nasopharyngeal cancer, tracheal cancer, laryngeal cancer, bronchial cancer , bronchiolar cancer, bladder cancer, esophageal cancer, stomach cancer, rectal cancer, colon cancer, prostate cancer, hollow organ cancer, bile duct cancer, urinary tract cancer, kidney cancer, uterine cancer, vaginal cancer and other gynecological adnexal cancer. The present invention also relates to methods of treating the above diseases. The present invention further relates to the use of the compound of formula 3 or a salt thereof to prepare a photodiagnostic agent for detecting the above-mentioned diseases and the following diseases, including: atherosclerosis, multiple sclerosis, diabetes, arthritis, rheumatism Arthritis, fungal infections, viral infections, chlamydial infections, bacterial infections, or parasitic diseases, HIV viral infections, hepatitis, herpes simplex, shingles, psoriasis, cardiovascular disease, and skin diseases. The present invention also relates to methods for detecting the above diseases using photodiagnostic agents. The present invention further relates to a method for low-temperature sterilization of surgical devices or other devices, including the steps of: providing a compound of Chemical Formula 3 or a salt thereof on the device; and subjecting the device to radiation treatment or sonication treatment. The invention further relates to a compound of formula 3 or a salt thereof linked or attached to a magnetic element. This compound acts as an MRI enhancer. The invention also relates to the use of such MRI enhancers for performing MRI scans.
Owner:PHOTO DIAGNOSTIC DEVICES PDD
H5, H7, H9 subtype auian flu virus real-time fluo rescent quantixative PCR detecting method
InactiveCN100378230CStrong specificityGood linear relationshipMicrobiological testing/measurementFluorescenceBio engineering
This invention relates to a real time fluorescence quantitative PCR test method for H5, H7 and H9 sub-avian flu viruses including the following steps: set-up of standard molecules, the design of specific virus quantitative PCR primer and the probes, pick-up of virus sample RNA, the inverse transcription polyenzyme chain reaction to the pic-up sample RNA to get a sample cDNA, the optimization and set-up of the PCR quantitative test method for M genes.
Owner:SHANGHAI JIAOTONG UNIV
Kit for real-time fluorescence RT-PCR detection of H9 subtype avian influenza virus
ActiveCN101736092AStrong specificityEfficacy monitoringMicrobiological testing/measurementSerum igeThroat swab
The invention relates to a kit for real-time fluorescence RT-PCR detection, in particular to a kit for early and rapid diagnosis of the infection of an H9 subtype avian influenza virus by using the one-step real-time fluorescence reverse transcription-polymerase chain reaction (RT-PCR) technology. Since the kit has high sensitivity and specificity, the rapid and early detection and quantitative analysis of the H9 subtype avian influenza virus in samples, such as the throat swab, the cloacae swab, the muscle or the viscera sample, the sera or the plasma and the like, can be realized by the kit.
Owner:DAAN GENE CO LTD
Colloidal gold immune chromatography fast differential diagnosis kit of chicken avian influenza vaccine immunity and virus strain infection and application
InactiveCN101012445AHigh biosecurityNo biohazardBacterial antigen ingredientsBacteriaStructural proteinVirus strain
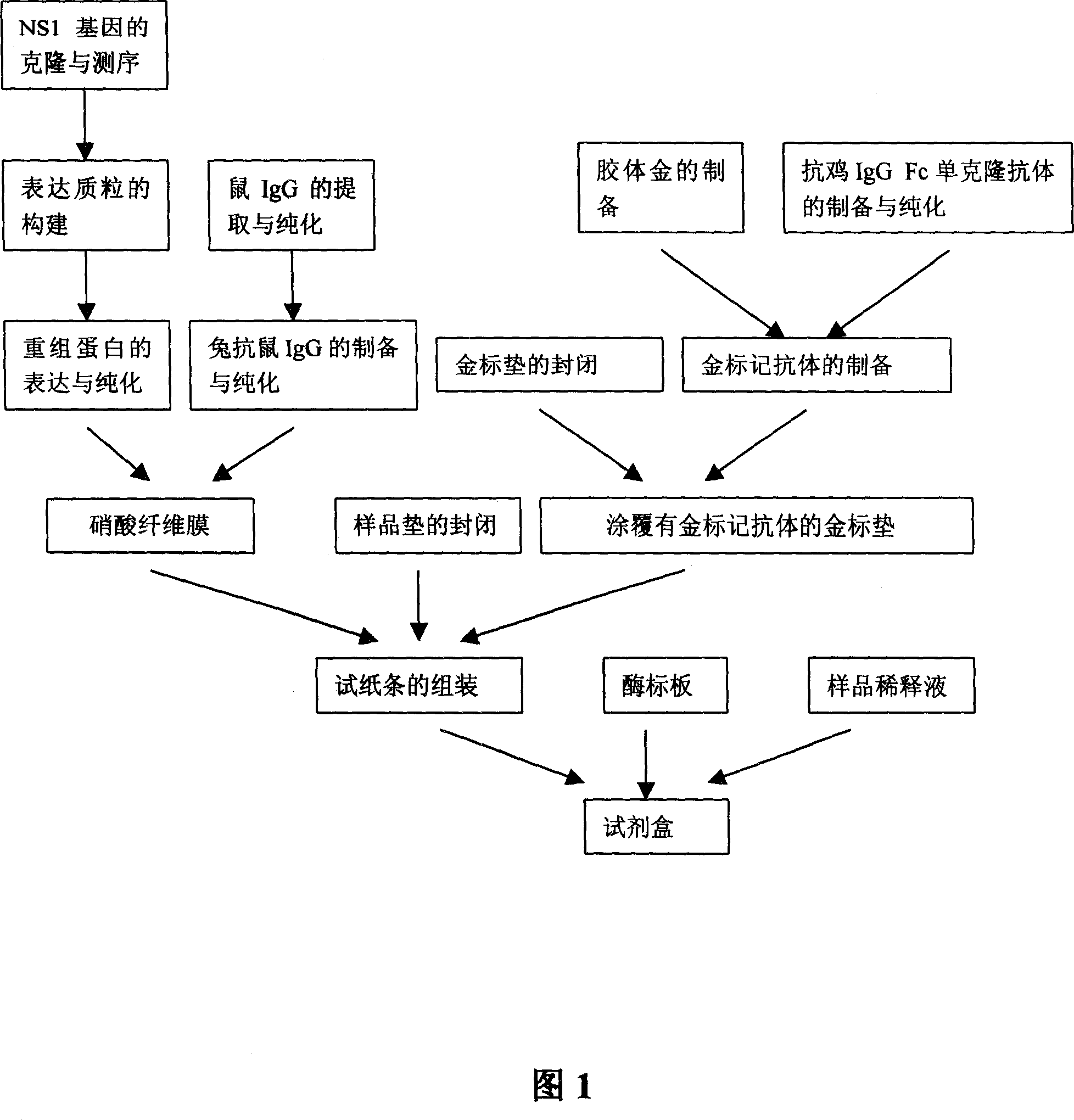
The invention discloses a rapid identifying diagnostic agent box and usage of flock influvaxin and virus and making method of BL21 / pKG-NS1 ( CCTCC NO: M207007) to express recombinant NS1 protein, which comprises the following parts: box, enzyme marking board, indicator paper and dilute liquid of sample, wherein the indicator paper is composed of adsorbing pad, cellulose nitrate film with detecting line of non-structural protein NS1 and quality control line of anti-mouse lgG, metal marking pad coated by anti-cock lgG Fc monoclone antibody, sample pad on the non-adsorbing support film sequently.
Owner:HUAZHONG AGRI UNIV
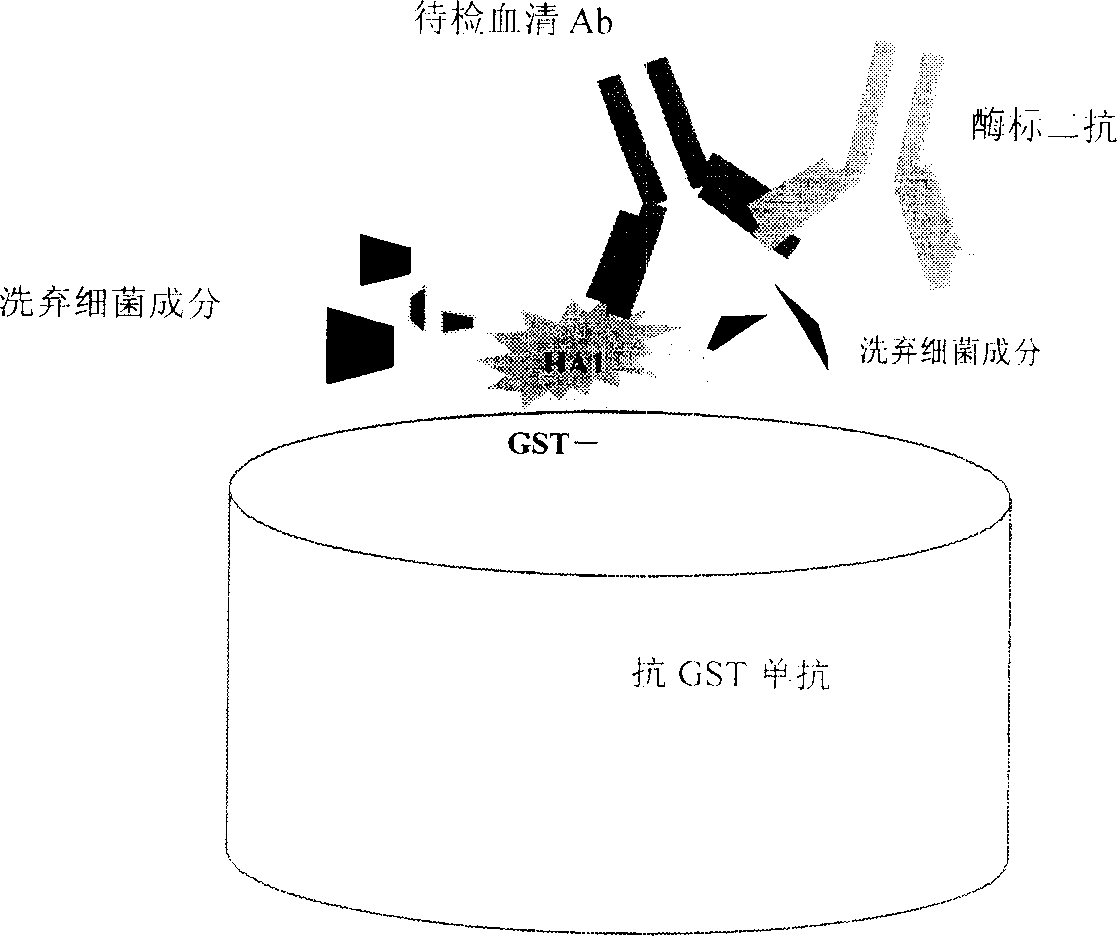
Detection method of aGST-ELISA of avian influenza virus (H5N1 subset) serum antibody
The invention relates to 'an aGST-ELISA detection method for bird flu viruses (H5N1 sub) serum antibodies', and its detection antigen is high- purity fusion protein GST-HA1 made by capturing fusion protein GST-HA1 on enzyme link plate by anti-GST monoantibody. And the method can be used to detect bird flu serum antibodies. And it has characters of simple antigen preparing process, high biosafety, low cost, easy to produce, etc, and lays the foundation of further development and research of bird flu detection reagent box.
Owner:北京翎羽生物科技有限公司
Detection kit of H9 subtype of avian influenza virus and application thereof
The invention discloses a detection kit of H9 subtype of avian influenza virus and an application thereof. The kit provided by the invention contains three pairs of primers, namely an inner primer pair, an outer primer pair and an annular primer pair which combine with the HA gene of the H9 subtype of avian influenza virus. The detection kit disclosed by the invention has high detection sensitivity, thus 100.5 EID50 of virus can be detected; and the detection kit is simple and convenient to operate and is especially suitable for the on-site clinical medical detection in primary-level and the food detection of H9 subtype of avian influenza virus.
Owner:CHINA AGRI UNIV
Siberian tiger alpha interferon and coding gene and application thereof
The invention discloses a Siberian tiger alpha interferon and a coding gene and application thereof. A Siberian tiger alpha interferon family is obtained by cloning a Siberian tiger genome, an amino acid sequence of its mature peptide is shown as SEQ ID No.1-11, and the nucleotide sequence of the coding gene is shown as SEQ ID No.12-22. A Siberian tiger IFN-alpha family protein has a shared conservative structure domain, namely an IFabd structure domain. Antiviral activity functional verification results prove that the Siberian tiger IFN-alpha family protein has the functions of resisting vesicular stomatitis virus, canine distemper and bird flu virus. The invention further discloses an antiviral drug composition, including an effective dose of the Siberian tiger alpha interferon for prevention or treatment and pharmaceutically acceptable excipients or carriers. A solid theoretical basis is laid for the Siberian tiger alpha interferon for the further study of other viral diseases and appearing of Siberian tiger alpha interferon products.
Owner:NORTHEAST FORESTRY UNIVERSITY
Enzyme-linked immunologic adsorption test method for identification and diagnosis of chicken infected with avian influenza
The invention relates to the enzyme combined immune absorbing testing for diagnosing bird flu affected chicks. It expands H9N2 bird flu virus, acquiring target section to connect with the carrier to restructure the expressing particle, injecting into bacillus coli expression to get a conjugated protein which is purified to get H9N2 bird flu virus NS1 protein to be antibody wrapping enzyme reaction plate, to build the antibody level to be reacted to inspect of the serum, getting the marginal value and least sample number of the enzyme combined immune adhesive test through statistics, and finally based on the average OD value to identify the bird flu affection status of the overall poultry. Through overall diagnosis, it alleviates nonspecific reaction, provides scientific proof for timely diagnosis and prevention of bird flu.
Owner:SHANGHAI JIAO TONG UNIV
Method of preparing polyvalent vaccine of viable bacteria
InactiveCN101130076AImprove stabilityImprove protectionAntiviralsAntibody medical ingredientsAntigenInfectious bronchitis
The invention discloses a making method of live polyvalent vaccine in the molecular biological domain, which protects five virus infections within one time through sucking and / or intaking expressive infectious bronchitis, marek's disease, facystic disease, newcastle disease and poultry influenza virus.
Owner:BEIJING GREAT GENIUS SCI & TECH DEV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com