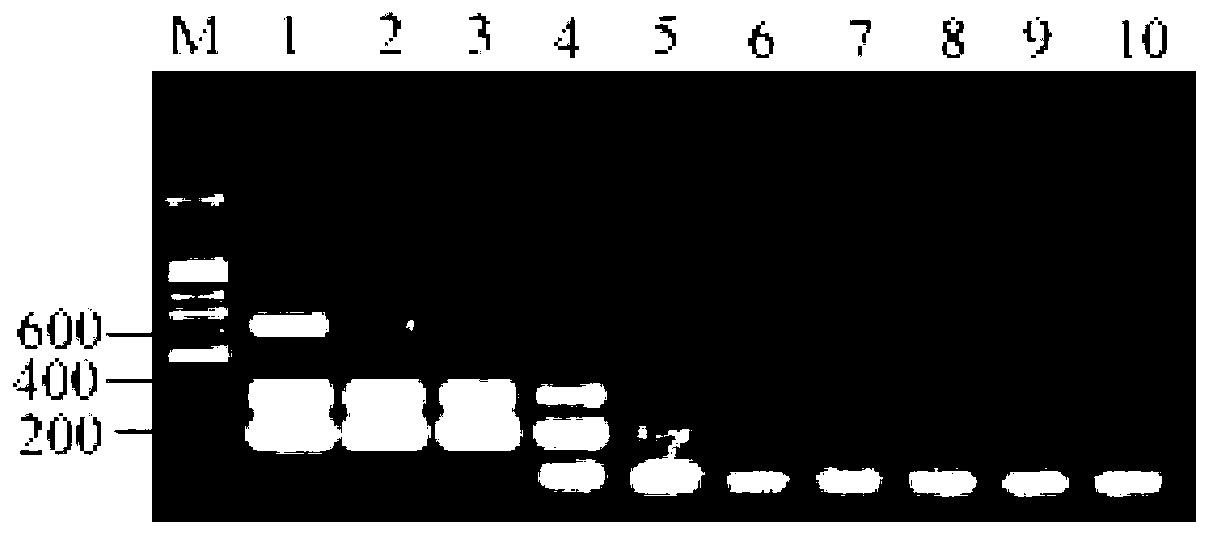Triple fluorescence quantitative reverse transcription-polymerase chain reaction (RT-PCR) detection kit for H7N9 avian influenza virus
A RT-PCR and avian influenza virus technology, applied in the field of H7N9 avian influenza virus triple RT-PCR detection kit, can solve the problems of lack of correction function of RNA polymerase, high frequency of gene mutation of avian influenza virus, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] The design of embodiment 1 primer and the preparation of kit
[0017] 1. Design of primers
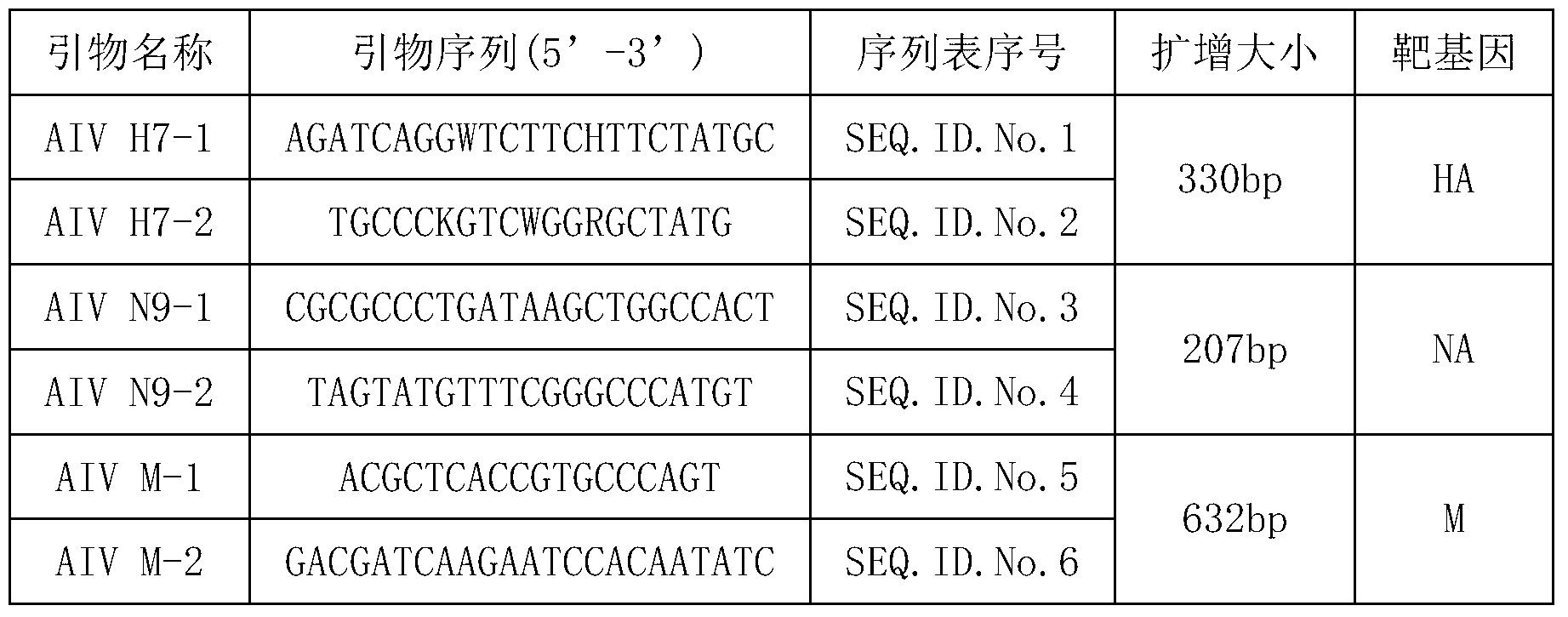
[0018] According to the conserved sequences of 3 genes including the HA gene of H7 subtype avian influenza virus, the NA gene of N9 subtype avian influenza virus and the M gene of all subtype avian influenza viruses published in GenBank, three genes were designed and synthesized through Blast verification. Pair-specific primers (Table 1).
[0019] Table 1 Primer Information
[0020]
[0021] AIV H7-1 and H7-2 primer pairs are used to detect whether it contains H7 subtype avian influenza virus;
[0022] AIV N9-1 and N9-2 primer pairs are used to detect whether it contains N9 subtype avian influenza virus;
[0023] The AIV M-1 and M-2 primer pairs are universal for the detection of all subtypes of avian influenza viruses.
[0024] 2. Sample preparation
[0025] According to the instructions of the TIANamp virus genome DNA / RNA extraction kit, avian influenza virus (H1N2, H3...
Embodiment 2 3
[0031] The specificity test of embodiment 2 triple RT-PCR
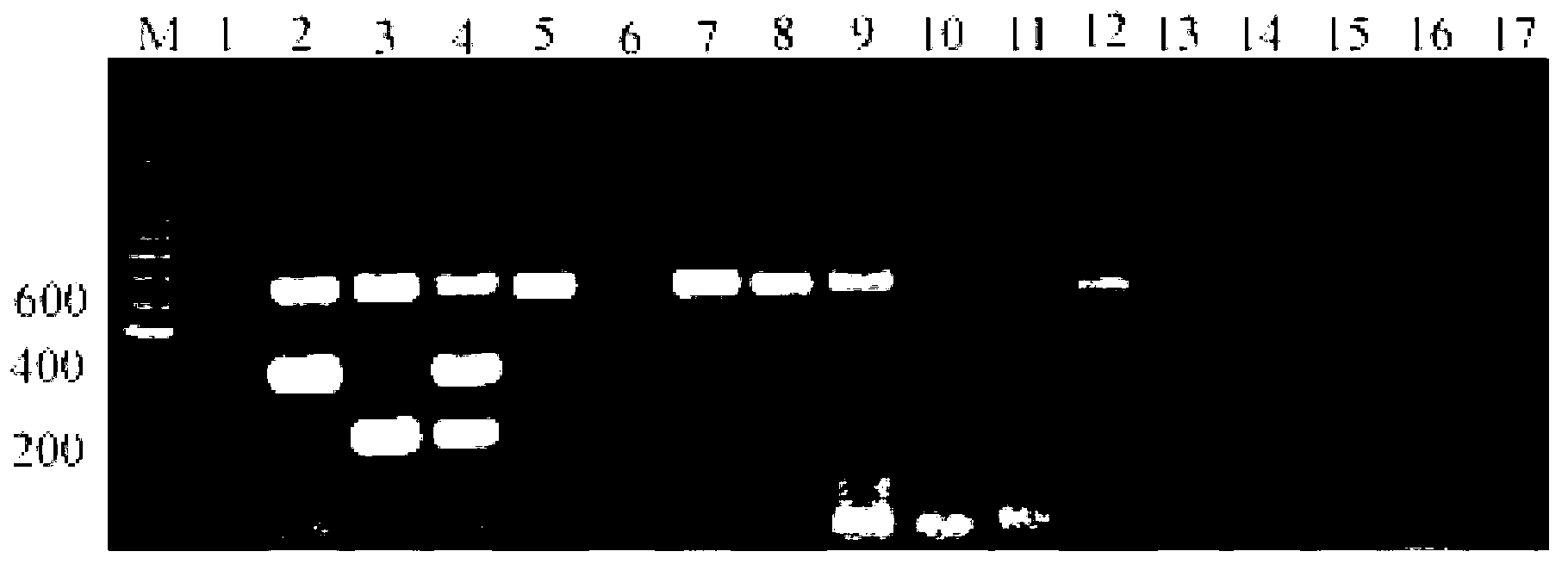
[0032] The cDNA of avian influenza virus (H7N2, H11N9, H7N9, H1N2, H1N7, H2N3, H3N2, H4N5, H5N3, H6N1, H8N4, H9N2 and H10N3), Newcastle disease virus, infectious bronchitis virus and infectious laryngotracheitis virus / DNA were added to the triple RT-PCR reaction system for amplification, and the specificity was tested. Applying optimal reaction conditions, RT-PCR amplification of avian influenza virus, Newcastle disease virus, infectious bronchitis virus and infectious laryngotracheitis virus nucleic acid was performed. Such as figure 1 As shown, viral nucleic acid templates containing H7 subtype AIV, N9 subtype AIV, H7N9AIV, and other subtypes of AIV can amplify amplified bands that match the size of the experimental design, while other common respiratory diseases in the same position do not. There are no amplified bands. The results showed that the detection of H7 subtype avian influenza virus, N9 subtype avian ...
Embodiment 3 3
[0033] Therefore, the primer pair set and method provided by the present invention can be applied to identify whether the test sample is infected with H7 subtype avian influenza virus, N9 subtype avian influenza virus, H7N9 avian influenza virus and other subtype avian influenza viruses. If a fragment of 330bp is obtained, the sample to be tested contains H7 subtype avian influenza virus, otherwise there is no; if a fragment of 207bp is obtained, the sample to be tested contains N9 subtype avian influenza virus, otherwise there is no; , 330bp and 632bp fragments, the sample to be tested contains H7N9 subtype avian influenza virus, and vice versa; Types of avian influenza virus, and vice versa. The sensitivity test of embodiment 3 triple RT-PCR
[0034] Use the full-length primers of H7 subtype avian influenza virus HA gene, N9 subtype avian influenza virus NA gene and avian influenza virus M gene full-length primers to carry out PCR amplification with the corresponding cDNA t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com