Blue fluorescent compound and preparation method thereof
A blue fluorescence and compound technology, applied in the field of fluorescent compounds, can solve the problems of poor water solubility and photostability, high toxicity and side effects, cumbersome synthesis methods, etc., and achieve high quantum yield, simple preparation method, and high cell survival rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
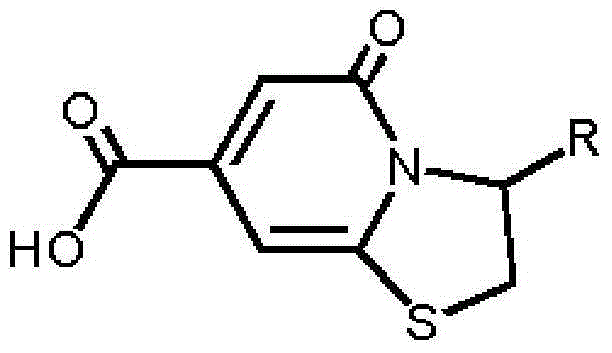
[0026] A preparation method of blue fluorescent compound: 10mmol of anhydrous citric acid and 1mmol of cysteine are added to a flask, 20mL of water is added to the flask to dissolve anhydrous citric acid and cysteine, and then The flask was placed in an oil bath, condensed and refluxed at 100°C for 12 hours; then naturally cooled to room temperature, added deionized water, left to stand at 0°C to precipitate solids, filtered, washed with water three times and placed in a vacuum drying oven Dry at 100°C to obtain a blue fluorescent compound (TPDCA).
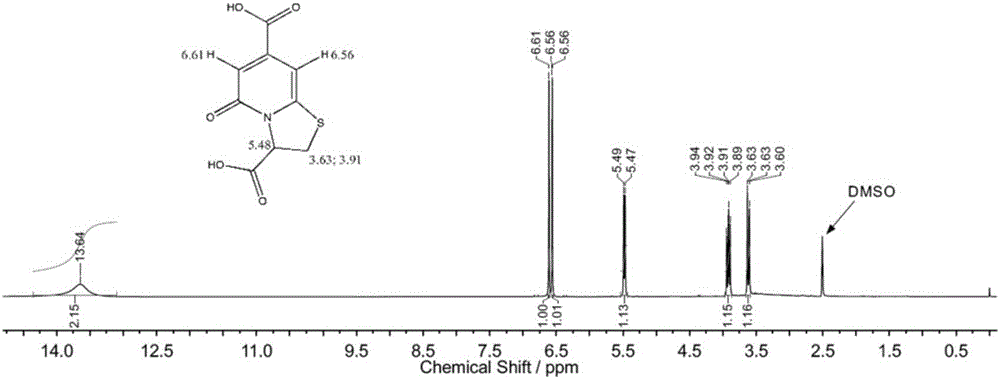
[0027] The structure of the above-mentioned blue fluorescent compound TPDCA is determined by nuclear magnetic resonance spectroscopy, see figure 1 , the peak at 13.6ppm is the hydrogen on the two carboxyl groups, the peak at 6.5-6.6ppm is the hydrogen of the double bond on the pyridone structure, and the peaks at 3.6 and 3.9ppm are the CH on the thiazole ring structure 2 The hydrogen of 5.4-5.5ppm is the hydrogen of CH on the t...
Embodiment 2
[0030] A preparation method of a blue fluorescent compound: 10mmol of citric acid containing crystal water and 10mmol of cysteamine are added to a flask, and 30mL of N,N-dimethylformamide is added to the flask to make citric acid, Dissolve cysteamine, then place the flask in an oil bath, condense and reflux at 150°C for 1 hour; take out the above flask, cool to room temperature naturally, add deionized water, stand at 5°C to precipitate solids, filter, The resulting precipitate was washed three times with water and then dried in a vacuum oven at 95° C. to obtain a blue fluorescent compound (TPCA).
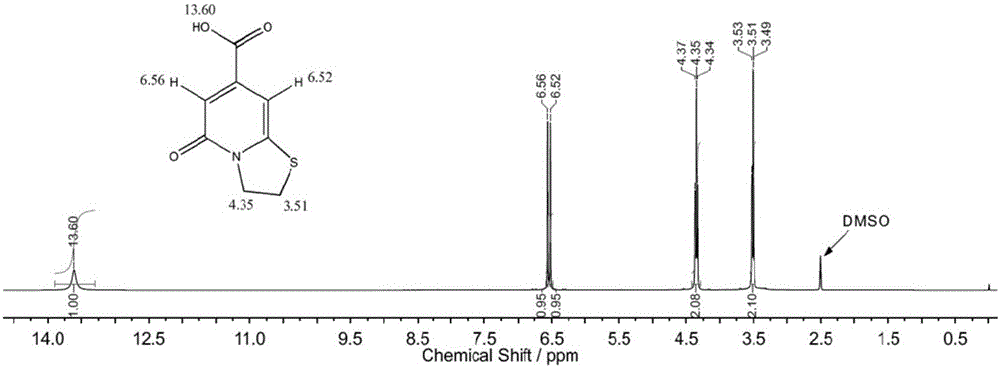
[0031] The structure of the above-mentioned blue fluorescent compound TPCA is determined by nuclear magnetic resonance spectroscopy, see figure 2 , the 13.6ppm peak is the hydrogen on the carboxyl group, the 6.5-6.6ppm peak is the hydrogen of the double bond on the pyridone structure, and the 3.5ppm peak is the CH adjacent to the sulfur atom on the thiazole ring structure 2 hydro...
Embodiment 3
[0034] A preparation method of a blue fluorescent compound: add 10mmol of anhydrous citric acid and 100mmol of cysteine to a flask, then add 50mL of dimethyl sulfoxide to the flask to make anhydrous citric acid, cysteine Dissolve the acid, then place the flask in an oil bath, condense and reflux at 150°C for 7 hours; then take out the flask, cool it down to room temperature naturally, add deionized water and let it stand at 2°C to precipitate solids, filter, and the obtained The precipitate was washed three times with water and then dried in a vacuum oven at 105° C. to obtain a blue fluorescent compound (TPDCA).
[0035] The structure of the above-mentioned blue fluorescent compound TPDCA is determined by nuclear magnetic resonance spectroscopy, see figure 1 , the peak at 13.6ppm is the hydrogen on the two carboxyl groups, the peak at 6.5-6.6ppm is the hydrogen of the double bond on the pyridone structure, and the peaks at 3.6 and 3.9ppm are the CH on the thiazole ring struc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com