Medicament composition for treating respiratory disease
A composition and respiratory technology, applied in the field of salt compositions containing glucocorticoids and leukotriene antagonists, can solve problems such as reducing adverse reactions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Montelukast 20g
[0042] Budesonide 10g
[0043] Ethanol 750g
[0044] Propylene glycol 150g
[0045] Dichlorodifluoromethane 1500g
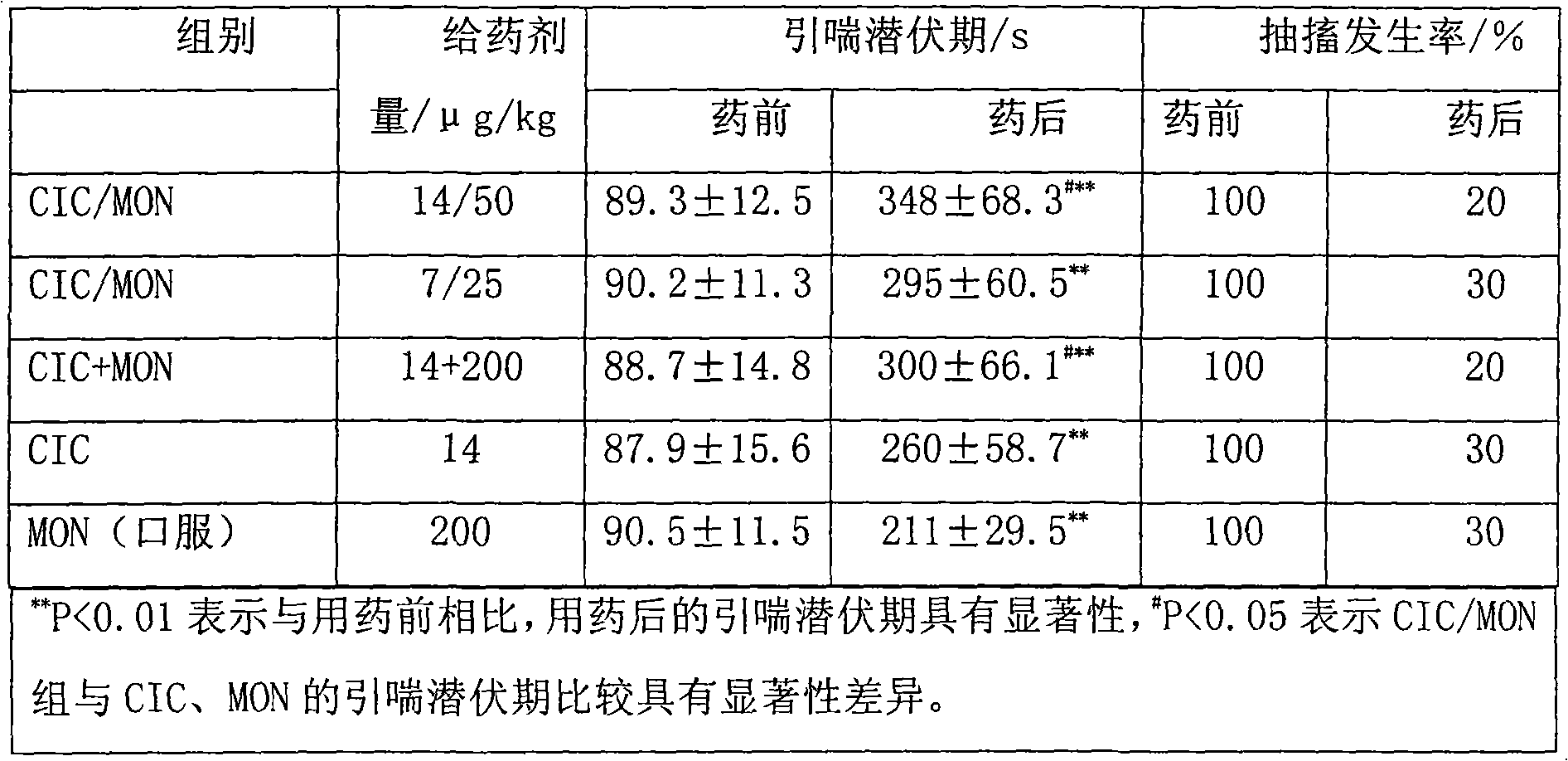
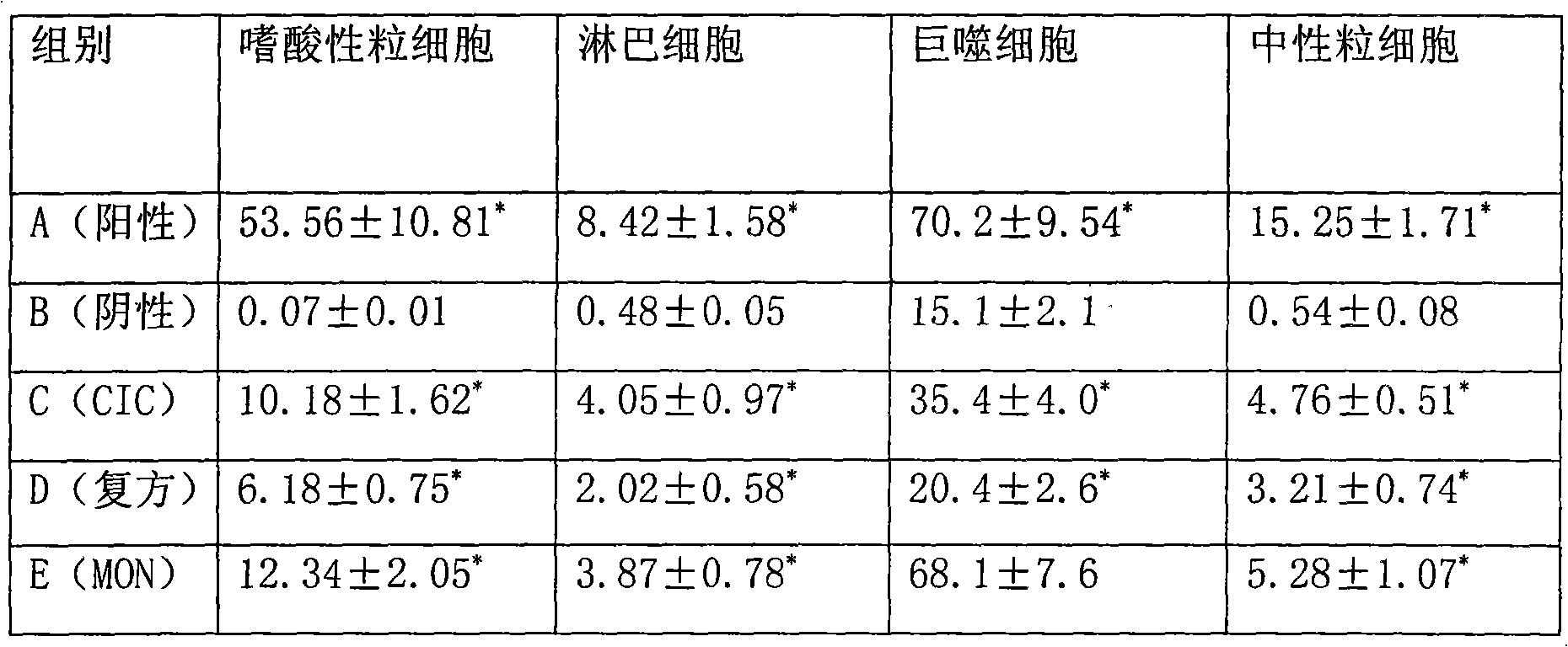
[0046] Preparation process: Add the prescribed amount of montelukast and budesonide into ethanol and propylene glycol that are evenly stirred, stir, heat in a warm water bath to dissolve the raw materials, filter with a sand core funnel, fill in divided doses, and seal the dose valve The system is respectively pressurized and injected with dichlorodifluoromethane to obtain the product. Theoretical filling is 1000 bottles, and the filling yield is above 85%. After 30 minutes of leak detection in a water bath at 45-50°C, there is no leakage. Each bottle is 50 presses, and each press contains 400 μg of montelukast and 200 μg of budesonide.
Embodiment 2
[0048] Montelukast Sodium 12.5g
[0049] Ciclesonide 3.5g
[0050] Ethanol 200g
[0051] Glycerol 19.5g
[0052] HFA227 750g
[0053] HFA134a 750g
[0054] Vitamin C 7.5g
[0055] Preparation process: add the prescribed amount of montelukast and ciclesonide into the well-stirred vitamin C, ethanol and glycerol, stir, heat in a warm water bath to dissolve the raw materials, filter through a sand core funnel, and pour in doses Packing and sealing the dosage valve system, respectively repressurizing HFA134a and HFA227 to obtain, theoretically canning 1000 bottles, and the filling yield is above 85%. After 30 minutes of leak detection in a water bath at 45-50°C, there is no leakage. Each bottle is 50 presses, and each press contains 250 μg of montelukast sodium and 70 μg of ciclesonide.
Embodiment 3
[0057] Montelukast 5g
[0058] Fluticasone Propionate 10g
[0059] Ethanol 750g
[0060] Glycerin 150g
[0061] Dichlorotetrafluoroethane 1500g
[0062] Sodium metabisulfite 7.5g
[0063] Preparation process: Add the prescribed amount of montelukast and fluticasone propionate into the well-stirred sodium metabisulfite, ethanol and glycerin, stir, heat in a warm water bath to dissolve the raw materials, filter with a sand core funnel, fill in divided doses, The dose valve system is sealed, and the dichlorotetrafluoroethane is respectively pressurized to obtain the product. The theoretical canning is 1000 bottles, and the filling yield is over 85%. After 30 minutes of leak detection in a water bath at 45-50°C, there is no leakage. Each bottle contains 50 presses, each press contains 100 μg of montelukast and 200 μg of fluticasone propionate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com