Brimonidine tartrate in situ forming eye gel
A technology of brimonidine tartrate and brimonidine ophthalmic technology is applied in the field of brimonidine tartrate ophthalmic ready-to-use gel, and can solve the problem of limiting the curative effect of brimonidine tartrate ophthalmic solution to be widely used, the retention time is short, and the The problem of large loss of eye drops, etc., achieves the effect of enriching the drug dosage form, prolonging the residence time, and having no adverse irritant reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
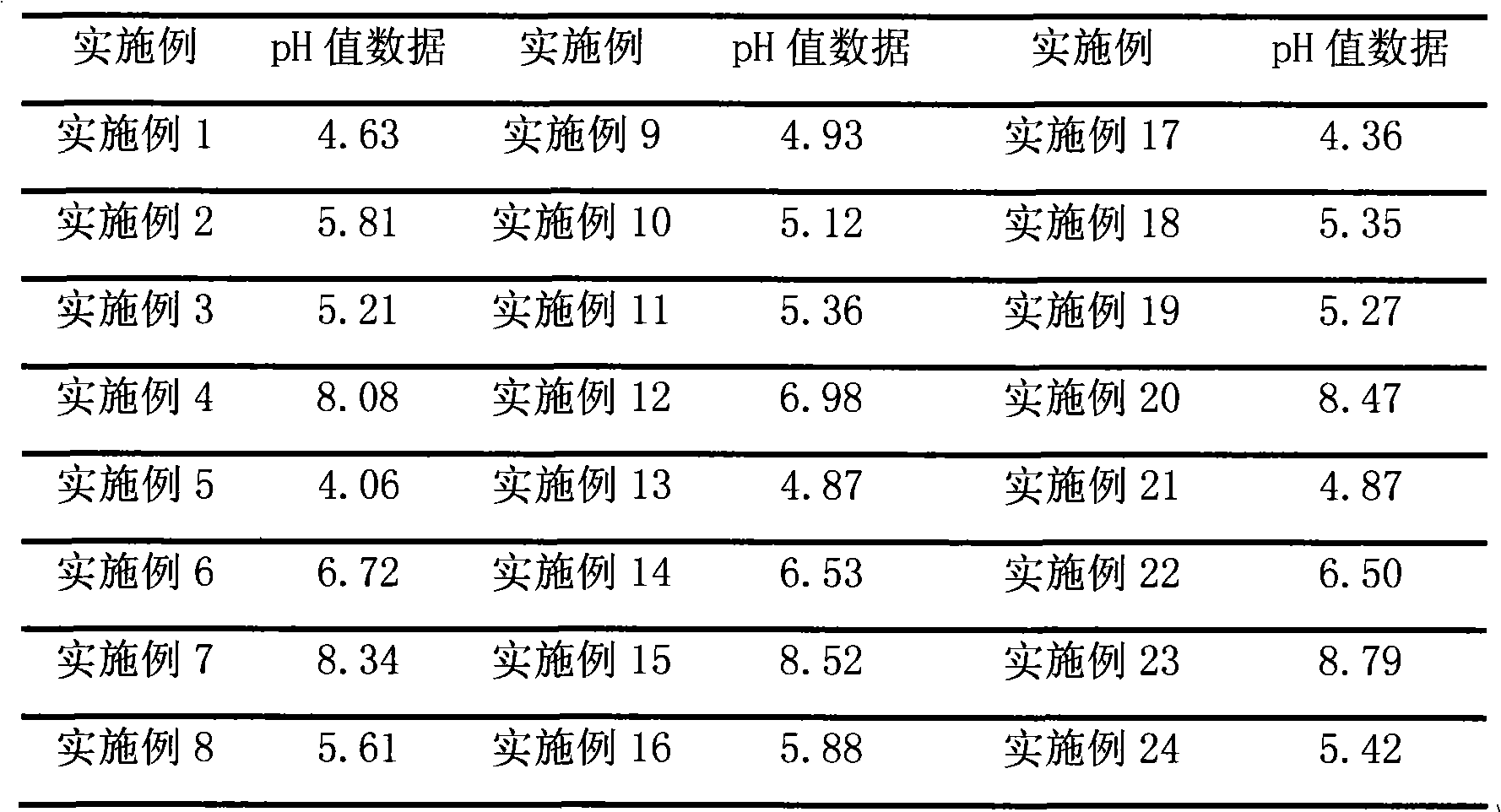
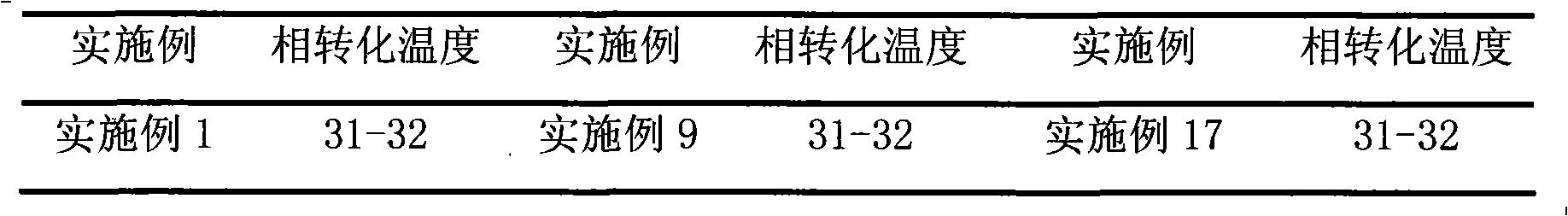
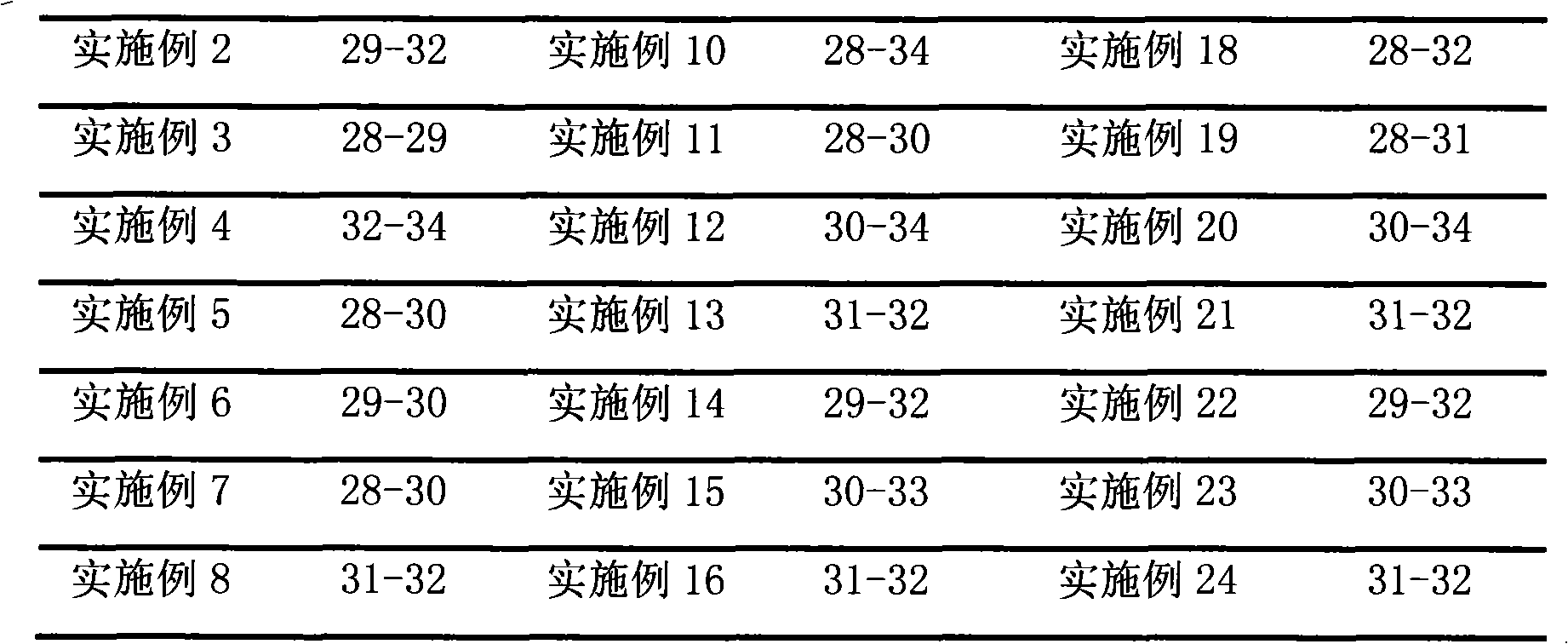
Examples
Embodiment 1
[0023] Prescription: 1 gram of brimonidine tartrate, 400 milliliters of hydrochloric acid (0.05mol / L)
[0024] Sodium Citrate 8.7g Methylcellulose 5.0g Phenylethyl Alcohol 0.5g
[0025] Ethanol 5ml Sodium Chloride 1.0g PEG-1000 20g
[0026] PEG-400 5g water for injection appropriate amount
[0027] Preparation method: Heat water for injection to boil, cool to 80°C, add brimonidine tartrate, dissolve phenylethyl alcohol in ethanol, add dropwise to the above solution while stirring, add sodium citrate, stir for more than 30 minutes, then add Stir after hydrochloric acid, filter through a microporous membrane (0.22 μm), heat the filtrate to 40°C, add sodium chloride, methylcellulose, PEG-1000 and PEG-400 while hot, and stir to room temperature (18-25 ℃), add water for injection to make up the weight to 1000 milliliters, stir to make it form a uniform gel, and aseptically pack it.
Embodiment 2
[0029] Prescription: 1.2 grams of brimonidine tartrate, 200 milliliters of sulfuric acid (0.05mol / L)
[0030] Sodium Citrate 8.7g Methylcellulose 5.0g Benzyl Alcohol 0.5g
[0031] Ethanol 5ml Sodium Chloride 2.0g PEG-1000 70g
[0032] Appropriate amount of water for injection
[0033] Preparation method: heat water for injection to boil, cool to 80°C, add brimonidine tartrate, dissolve benzyl alcohol in ethanol, add dropwise to the above solution while stirring, add sodium citrate, stir for more than 30 minutes, then add Stir after sulfuric acid, filter through a microporous membrane (0.22μm), heat the filtrate to 40°C, add sodium chloride, methylcellulose and PEG-1000 while hot, stir to room temperature (18-25°C), and then Add water for injection to make up the weight to 1000 ml, stir to make it form a uniform gel, and aseptically pack it.
Embodiment 3
[0035] Prescription: 1.3 grams of brimonidine tartrate, 200 milliliters of sodium hydroxide (0.1mol / L)
[0036] Boric acid 5.6g Methylcellulose 5.0g Sodium benzoate 0.5g
[0037] Ethanol 5ml Sodium Chloride 5.0g PEG-4000 100g
[0038] Appropriate amount of water for injection
[0039] Preparation method: Heat water for injection to boil, cool to 80°C, add brimonidine tartrate, add sodium citrate, sodium hydroxide and sodium benzoate, stir for more than 30 minutes, then add boric acid and stir, pass through a microporous membrane (0.22μm) filtered, the filtrate was heated to 40°C, PEG-4000 and methylcellulose were added while it was hot, stirred to room temperature (18-25°C), added water for injection to make up the weight to 1000 ml, stirred to form Uniform gel, aseptically dispensed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com