Composite medicinal preparation containing inosine matters
A preparation, the technology of inosine, which is applied in the field of combined pharmaceutical preparations containing inosine substances, can solve the problems of low drug compliance, pollution, and large dosage of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
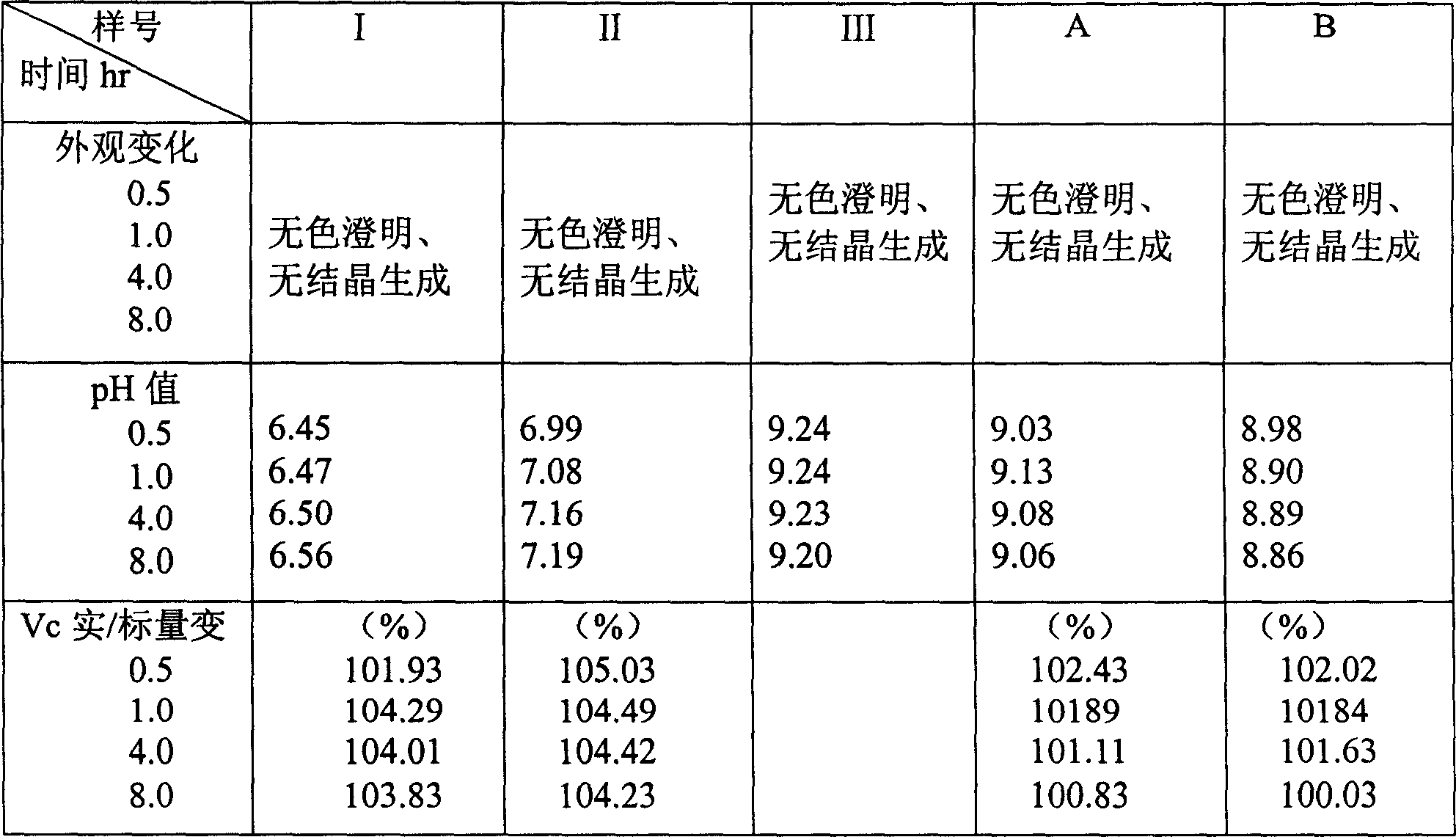
[0019] - control sample
[0020] Control sample I: Weigh 1.20 grams of vitamin C, add 10% glucose injection to 500 milliliters and shake well,
[0021] Control sample II: Weigh 2.40 grams of vitamin C, add 10% glucose injection to 500 ml and shake well,
[0022] Control sample III: Weigh 0.6 g of inosine, add 10% glucose injection to 500 ml and shake well,
[0023] - for samples
[0024] For sample A: weigh 1.20 grams of vitamin C, weigh 0.6 grams of inosine, add 10% glucose injection to 500 ml and shake well,
[0025] For sample B: weigh 2.40 grams of vitamin C, weigh 0.6 grams of inosine, add 10% glucose injection to 500 ml and shake well,
[0026] Use the above-mentioned control sample and the sample for the paper chromatography composition test as follows.
[0027] --Paper chromatography composition test:
[0028] Chromatographic filter paper: No. 1 medium speed,
[0029] Developing solvent: n-butanol-glacial acetic acid-purified water,
[0030] Chromogen: 254nm ult...
Embodiment approach 2
[0037] Freeze-dried powder injection:
[0038] Ingredient Vial Dose (mg)
[0039] active ingredient
[0040] Inosine 100
[0041] Vitamin C 100
[0042] The finished product is obtained by following the freeze-dried powder injection preparation process: take 100 g of injection-grade mannitol, add 500 ml of water for injection to dissolve. Weigh inosine 100g, Vc100g and dissolve in the above solution. Add 0.25g of activated carbon for injection, heat to 60°C-80°C, stir for 20 minutes, coarsely filter and decarbonize, wash the filter with an appropriate amount of water for injection, combine the filtrate, add water for injection to 1900ml, measure the pH value of the solution, and use Hydrochloric acid solution or sodium hydroxide solution to adjust the pH value of the solution to 5.5-6.0, add water for injection to the full amount, measure the pH value and content of the solution, control the pH value between 5.5-6.0, and then use 0.22 Sterilize by microporous ...
Embodiment approach 3
[0044] tablet:
[0045] Ingredient Vial Dose (mg)
[0046] active ingredient
[0047] Inosine 200
[0048] Vitamin C 100
[0049] medical supplements
[0050] powdered sugar 50
[0051] Starch 120
[0052] Low-substituted hydroxypropyl cellulose 10
[0055] According to tablet preparation standard process operation, the finished product is obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com