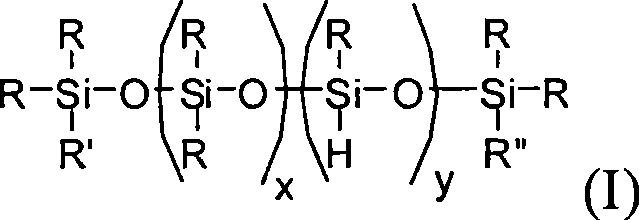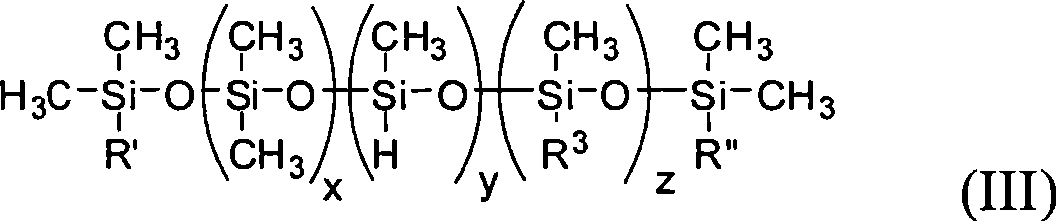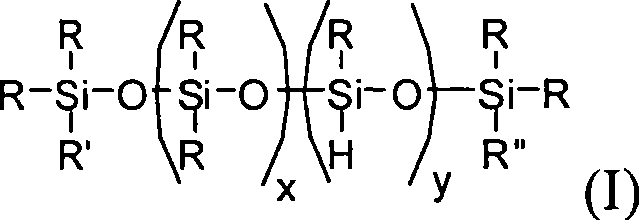Method for producing branched polyorganosiloxane
A technology of branched siloxane and siloxane, which is applied in the field of preparation of branched polyorganosiloxane, can solve problems such as complex silane, and achieve the effect of convenient processing and handling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0067] These branched siloxanes can also be prepared by combining the substrate to be hydrolyzed (E-A-B-L) with a suitable The hydrosilylation catalyst was added directly to the reaction mixture.
[0068] The siloxanes of the invention, especially those having the group -A-B-L, are especially useful in the preparation of polyurethane foams, preferably as foam stabilizers, and in the preparation of defoamer preparations.
Embodiment 1
[0084] has an average structure (CH 3 ) 3 SiO-[(CH 3 ) 2 SiO] 20 -[(CH 3 )HSiO] 5 -Si(CH 3 ) 3 The reaction of siloxane with cesium laurate
[0085] 40.5 g of this siloxane were first added to a 250 ml three-necked flask equipped with a stirrer, jacketed coil condenser and thermometer. At 50°C, 0.2 g of cesium laurate was added, then warmed to 130°C and stirred at 130°C for another 1 h. Gas volumetric hydrogen measurements yielded a 21% reduction in silane hydrogen. A yellowish, viscous product was obtained which 29 Si-NMR spectra (prepared with a Brucker AVANCE 400 NMR spectrometer with XWIN-NMR 3.1 evaluation software and tetramethylsilane as internal standard) show chemical shift signals from 10 to 7 ppm (M-units; 6.5% of total signal intensity %), -5 to -7ppm (M'-unit; 5.1% of total signal intensity), -18 to -23ppm (D-unit; 72.7% of total signal intensity), -35 to -38ppm (D'-unit ; 6.4% of total signal intensity) and -63 to -69 ppm (T-unit; 9.3% of total signal...
Embodiment 2
[0087] has an average structure (CH 3 ) 3 SiO-[(CH 3 ) 2 SiO] 20 -[(CH 3 )HSiO] 5 -Si(CH 3 ) 3 The reaction of siloxane with cesium laurate and subsequent hydrosilylation
[0088] 40.5 g of this siloxane were first added to a 250 ml three-necked flask equipped with a stirrer, jacketed coil condenser, dropping funnel and thermometer. At 50° C., 0.2 g of cesium laurate was added, the temperature was raised to 130° C. and stirred at 130° C. for another 1 h. Gas Volume Hydrogen Determination Silane Hydrogen was reduced by 21%. The reaction mixture was cooled to 88° C. and Karstedt catalyst (platinum-divinyltetramethyldisiloxane complex from ABCR, 15 ppm mass platinum based on the total weight of the batch) was added. At 88° C., 88 g of a pure propylene oxide-containing polyether (average molar mass 1000 g / mol) started with allyl alcohol and terminated with methyl groups were added dropwise. Afterwards, it was stirred at 88° C. for another 1.5 h, and then the mixture was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com