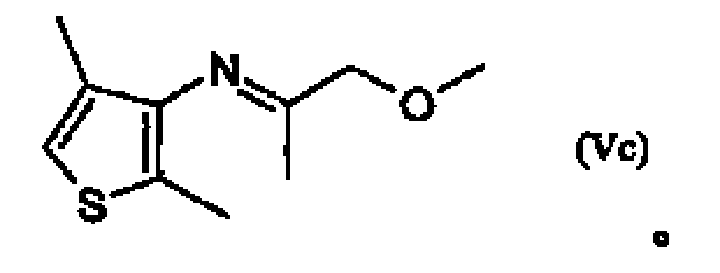
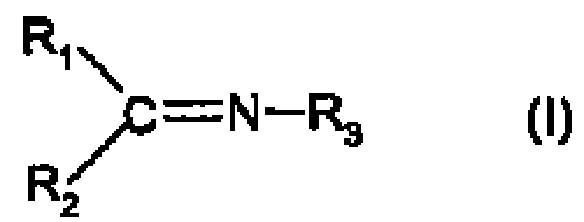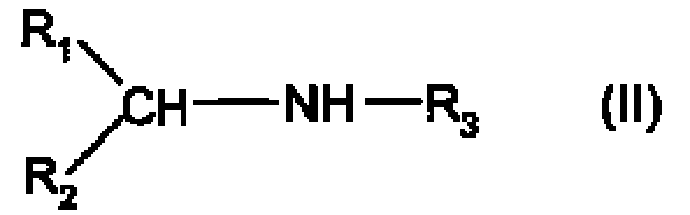Process for the hydrogenation of imines
An imine and hydrogenation technology, applied in organic chemistry methods, chemical instruments and methods, organic chemistry, etc., can solve the problems of low catalyst productivity and inability to achieve complete conversion, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0172] The following examples illustrate the synthesis of some phosphonium halides (see, Beller et al., Synthesis 2004, 934-941).
[0173] Synthesis of Diadamantylbenzylphosphonium Bromide
[0174] Diadamantylphosphine (10 mmol, 3.02 g) was suspended in 6 ml benzyl bromide and 40 ml dibutyl ether in air. The reaction mixture was stirred at 138 °C for 16 h. After cooling, the solid was filtered, washed with MTBE, and dried. Yield: 4.05 g, 86% (white powder).
Embodiment 2
[0176] Hydrogenation of 2,4,6-trimethyl-N-(4-methylpentan-2-yl (4-methylpentan-2-ylidene))aniline using different phosphonium halides and ligands (for comparison, also carried out To use Bu 4 Some experiments with NI):
[0177] The required additives (0.004-0.006 mmol) were weighed in a 1.5 ml GC-flask under argon (glove box). Then, 300 µl of toluene was added. Subsequently, 50 μl of 0.01 M [Ir(1,5-cyclooctadiene)Cl] in toluene was added 2 solution (0.0005 mmol) and 50 μl of 0.02 M ligand solution (0.001 mmol) in toluene. The mixture was stirred at room temperature for 15 minutes. Then, 250 μl neat substrate or 200 μl 2M or 1M substrate solution in toluene were added. The flask was closed with a septum which was pierced several times with a needle and placed on an aluminum microtiter plate and placed in an autoclave. The autoclave was flushed with hydrogen and 55 bar of hydrogen was introduced. The temperature was set at 65 °C and stirred for 1.75 h. The pressure was r...
Embodiment 3
[0180] The following examples were carried out in a similar manner to Example 2, but with varying ligands, solvents, reaction times, reaction temperatures and / or phosphonium halides:
[0181]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com