Method for synthesizing alpha-MnO2 micrometre hollow sphere and nanocluster
A technology of nano-clusters and hollow spheres, which is applied in the direction of manganese oxide/manganese hydroxide, etc., can solve the problems of long reaction time, and achieve the effect of mild reaction conditions and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
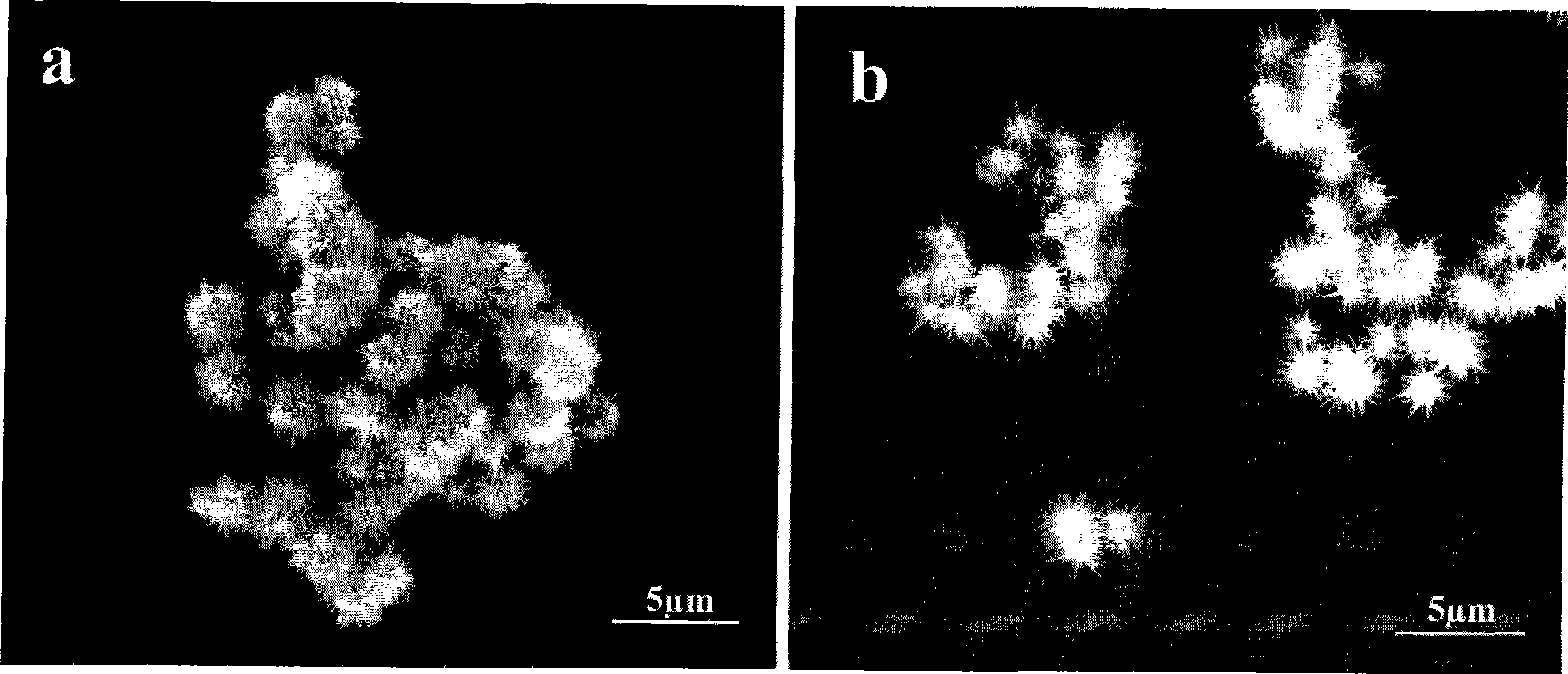
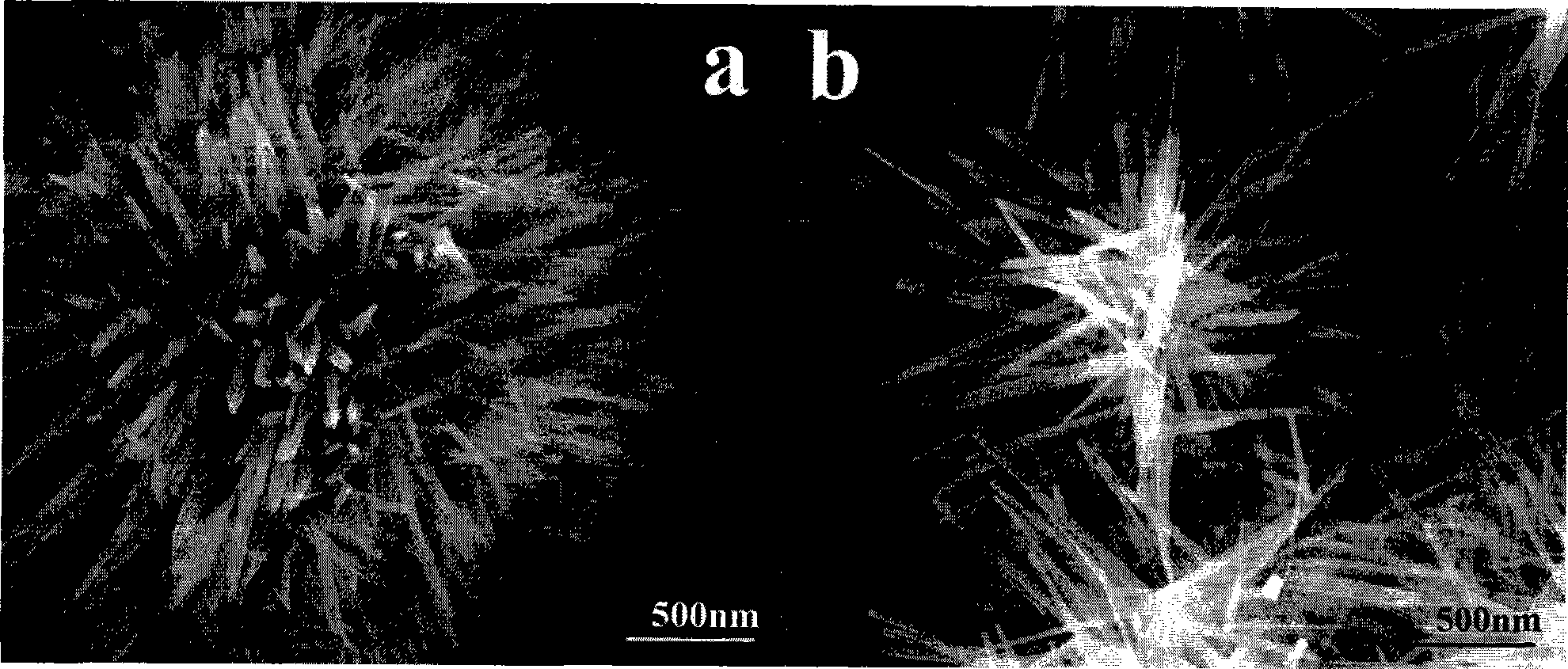
[0022] Weigh 99.5% potassium persulfate according to the stoichiometric ratio of 1:1, which is analytically pure; 99% manganese sulfate monohydrate, which is analytically pure; add the above raw materials into the hydrothermal reaction kettle, add deionized water and stir fully to make dissolve it; measure 2 mL of concentrated sulfuric acid with a mass fraction of 95-98% and add it to a hydrothermal reaction kettle. At the beginning of the reaction, the concentration of sulfuric acid was 1mol / L, and the concentration of potassium persulfate and manganese sulfate was 0.05mol / L. The hydrothermal reaction vessel was placed in an oven at a constant temperature of 110° C. for 6 h, and then cooled to room temperature naturally. After the reaction product was centrifuged and washed 5 times with deionized water and absolute ethanol, it was checked that it did not contain SO 4 2- After drying at 60°C in the air atmosphere, the obtained product is α-MnO 2 micron hollow spheres. figu...
Embodiment 2
[0024] Weigh 99.5% potassium persulfate according to the stoichiometric ratio of 1:1, which is analytically pure; 99% manganese sulfate monohydrate, which is analytically pure; add the above raw materials into the hydrothermal reaction kettle, add deionized water and stir fully to make dissolve it; measure 2 mL of concentrated sulfuric acid with a mass fraction of 95-98% and add it to a hydrothermal reaction kettle. At the beginning of the reaction, the concentration of sulfuric acid was 1mol / L, and the concentration of potassium persulfate and manganese sulfate was 0.05mol / L. The hydrothermal reaction vessel was placed in an oven at a constant temperature of 110°C for an hour and then naturally cooled to room temperature. After the reaction product was centrifuged and washed 5 times with deionized water and absolute ethanol, it was checked that it did not contain SO 4 2- After drying at 60°C in the air atmosphere, the obtained product is α-MnO 2 micron hollow spheres.
Embodiment 3
[0026] Weigh 99.5% potassium persulfate according to the stoichiometric ratio of 1:1, which is analytically pure; 99% manganese sulfate monohydrate, which is analytically pure; add the above raw materials into the hydrothermal reaction kettle, add deionized water and stir fully to make dissolve it; measure 2 mL of concentrated sulfuric acid with a mass fraction of 95-98% and add it to a hydrothermal reaction kettle. At the beginning of the reaction, the concentration of sulfuric acid was 1mol / L, and the concentration of potassium persulfate and manganese sulfate was 0.05mol / L. The hydrothermal reaction kettle was placed in an oven at a constant temperature of 110°C for 12 hours, and then cooled to room temperature naturally. After the reaction product was centrifuged and washed 5 times with deionized water and absolute ethanol, it was checked that it did not contain SO 4 2- After drying at 60°C in the air atmosphere, the obtained product is α-MnO 2 micron hollow spheres.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com