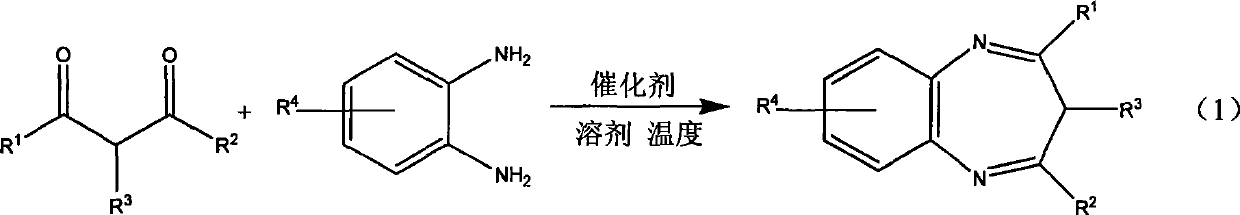
Method for synthesizing diaza-onium salt
A synthetic method, diazepine technology, applied in the direction of organic chemistry, can solve the problems of long reaction time, potential safety hazards, unfriendly environment, etc., and achieve the effect of simple operation, mild conditions, and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
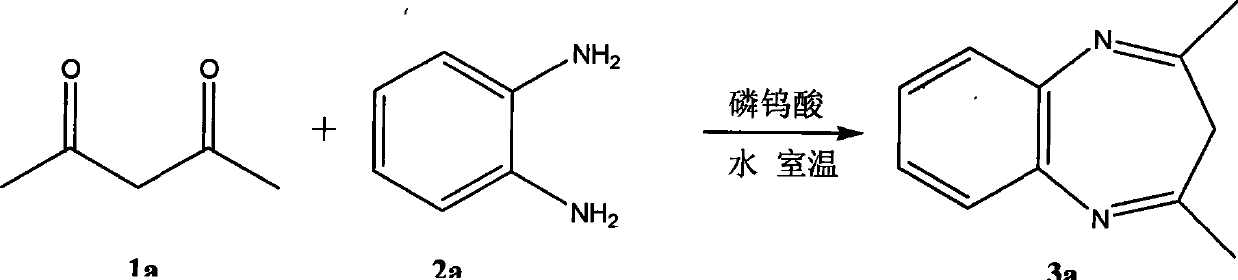
[0018]
[0019] Dicarbonyl compound 1a (5mmol, 500mg) and substituted o-phenylenediamine compound 2a (5.5mmol, 594mg) in pure water (6ml), add 0.005 equivalent of phosphotungstic acid (0.025mmol, 72mg) and stir at 25°C (ie room temperature) The reaction was carried out for 12 hours. After the reaction was completed, the reaction solution was extracted with ethyl acetate, the organic layers were combined, the liquid was separated and the water layer was discarded; the organic layer was washed successively with saturated sodium carbonate solution and saturated sodium chloride solution, dried over anhydrous magnesium sulfate, filtered, and precipitated under reduced pressure. A crude product was obtained; the crude product was separated by column chromatography to obtain a light yellow solid 3a (518 mg, 72%).
[0020] Attached: 3a: 1 H NMR (CDCl 3 ): d 2.34 (s, 6H, CH 3 ), 2.81 (s, 2H, CH 2 ), 7.20-7.22 (m, 2H, Ph), 7.35-7.38 (m, 2H, Ph). 13 C NMR (CDCl 3 ): d 27.87, 43....
Embodiment 2
[0022]
[0023] Dicarbonyl compound 1a (5mmol, 500mg) and substituted o-phenylenediamine compound 2a (5.5mmol, 594mg) in pure water (6ml), add 0.01 equivalent of phosphotungstic acid (0.05mmol, 114mg) and stir at 25°C (ie, room temperature) React for 12 hours. After the reaction was completed, the reaction solution was extracted with ethyl acetate, the organic layers were combined, the liquid was separated and the water layer was discarded; the organic layer was washed successively with saturated sodium carbonate solution and saturated sodium chloride solution, dried over anhydrous magnesium sulfate, filtered, and precipitated under reduced pressure. A crude product was obtained; the crude product was separated by column chromatography to obtain a light yellow solid 3a (662 mg, 92%).
Embodiment 3
[0025]
[0026] Dicarbonyl compound 1a (5mmol, 500mg) and substituted o-phenylenediamine compound 2a (5.5mmol, 594mg) in pure water (6ml), add 0.015 equivalent of phosphotungstic acid (0.075mmol, 216mg) and stir at 25°C (ie, room temperature) React for 12 hours. After the reaction was completed, the reaction solution was extracted with ethyl acetate, the organic layers were combined, the liquid was separated and the water layer was discarded; the organic layer was washed successively with saturated sodium carbonate solution and saturated sodium chloride solution, dried over anhydrous magnesium sulfate, filtered, and precipitated under reduced pressure. A crude product was obtained; the crude product was separated by column chromatography to obtain a light yellow solid 3a (662 mg, 92%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com