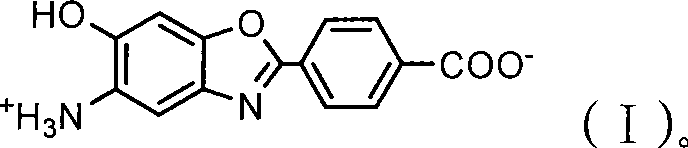
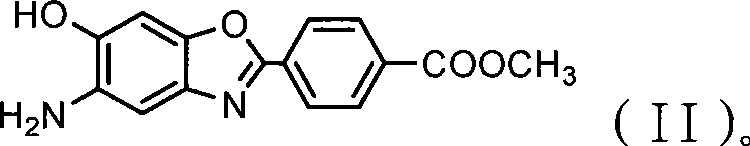
Use of 5-amino-6-hydroxy-2-(p-carboxyphenyl)benzoxazole salt
A technology for p-carboxyphenyl, benzoxazole carboxylammonium salt, which is applied in the field of preparing poly-p-phenylene benzobisoxazole resin, can solve the problems of affecting the molecular weight of PBO, lack of practicability, low yield and the like, and achieves no Gas interference and safety hazards, excellent anti-oxidative stability, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: MAB alkaline hydrolysis synthetic ABAS
[0038] 3g Na 2 CO 3 Dissolve in 100ml of water solvent, take MAB 2.00g (98.69%, 0.0069mol) into the mixture, pass N 2 Replace the air, carry out the hydrolysis reaction at 60°C until the solution is clear, filter while it is hot, add NaHSO to the filtrate after cooling 3 The saturated aqueous solution was adjusted to pH 6.5 to precipitate precipitates, filtered, washed with ice water, and dried in vacuo to obtain 1.61 g (0.0059 mol) of the khaki crystalline product ABAS monomer, with a purity of 99.28%. Qualitative FT-IR, 13 C-NMR, 1 H-NMR attribute analysis and elemental analysis, determined to be a new monomer of salt type AB type PBO: that is, 5-amino-6-hydroxyl-2-(p-carboxyphenyl) benzoxazole carboxyammonium salt ABAS monomer, received The rate is 85.2%.
[0039] FT-IR (KBr, cm -1 ): 3334.3, 3267.8, 3146.3, 1675.8 (carboxyammonium salt absorption peak), 1618.0, 1581.3, 1466.6, 1410.7, 1380.8, 1294.0, 1178....
Embodiment 2~5
[0042] Adopt the same operation of embodiment 1, get different parameters (two kinds of alkaline substances and proportioning, different raw material purity) by the parameter scope in table 1 and test, the results are shown in table 1:
[0043] Table 1 Preparation of ABAS monomer by hydrolysis of MAB (NaHSO 3 Adjust pH 6.5 to precipitate)
[0044]
[0045] a is K 2 CO 3 The consumption (g), embodiment 4 and embodiment 5 are K 2 CO 3 Hydrolysis process
[0046] For the hydrolysis of MAB, as can be seen from the data of embodiment 2,3, adopt Na 2 CO 3 During the hydrolysis process, the MAB raw material with lower purity (88.4-94.1%) can still obtain ABAS monomer with higher purity above 96% after hydrolysis. Using K 2 CO 3 The hydrolysis process is as in Example 4, only MAB with a purity of 94.1% can obtain 98.3% ABAS. It can be seen that while MAB is hydrolyzed, the resulting monomer ABAS can be refined and purified.
Embodiment 6~8
[0048] Adopt the same operation of embodiment 1, choose Na 2 CO 3 Hydrolysis process, by NaHSO of the present invention 3 Adjust the parameter scope of pH (get different parameter pH value 6.5,7.0,7.5) to react (the embodiment 7 of pH=7.5 is as comparative example), the results are shown in Table 2:
[0049] Table 2 Effect of pH on the precipitation of ABAS monomers prepared by hydrolysis of MAB
[0050]
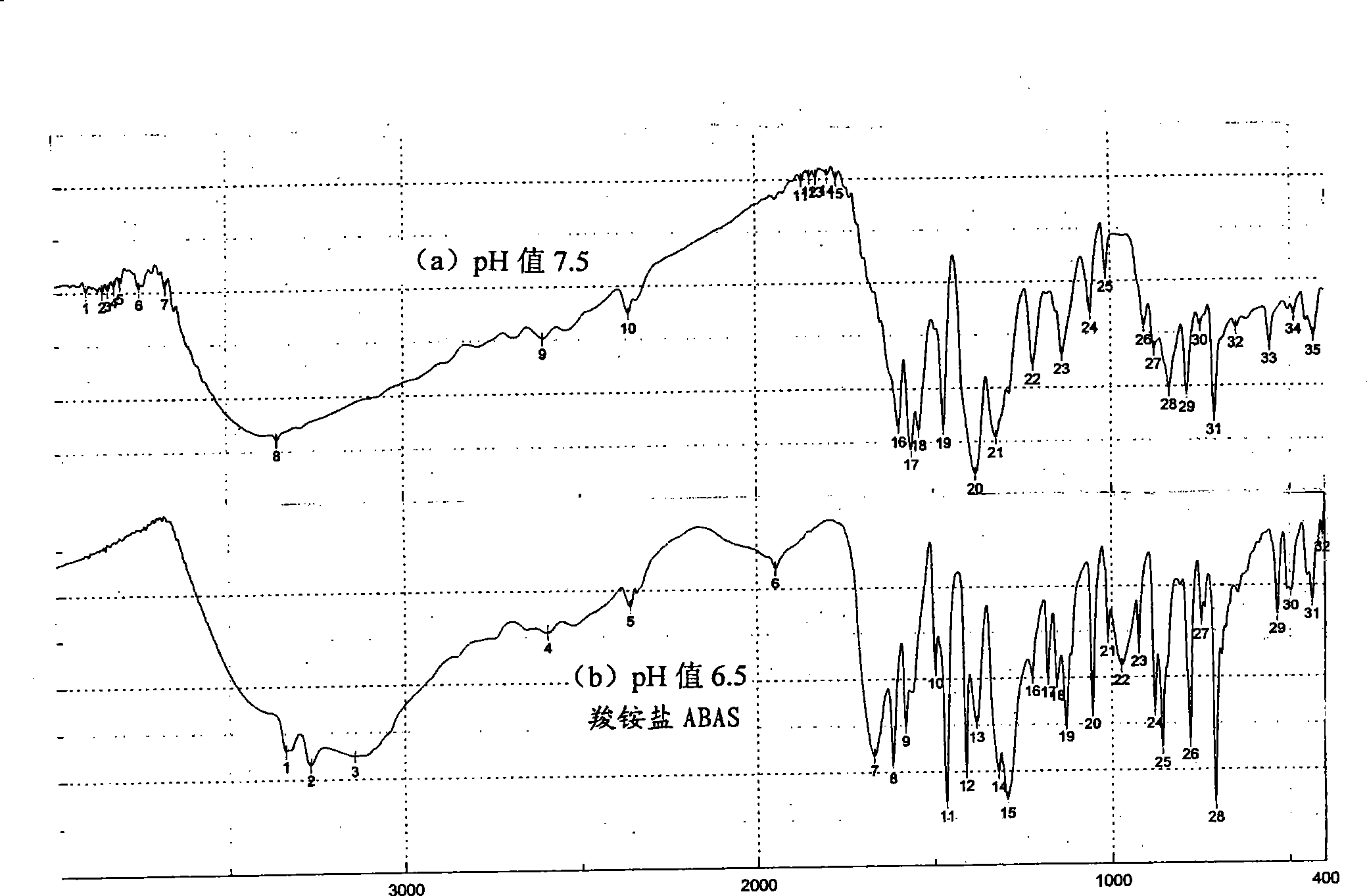
[0051] Wherein, the monomer that embodiment 6 adjusts pH value 7.0 makes and the ABAS infrared absorption that embodiment 1 adjusts pH 6.5 and IR figure are completely consistent; And when embodiment 7 adjusts pH value 7.5, separate out its infrared absorption of ABA monomer The IR pattern is completely different. Put the substance of this different IR pattern back into the water and then use NaHSO 3 Adjust the pH value to 6.5, and the IR pattern of the obtained monomer is completely the same as that of ABAS (see Example 8). Their infrared absorption is relatively fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com