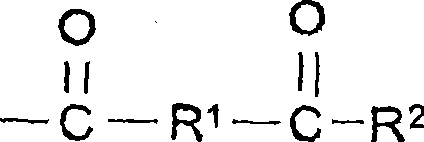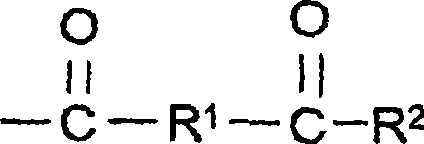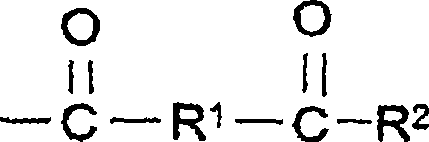Aqueous coating compositions containing acetoacetyl-functional polymers, coatings, and methods
A technology of acetoacetyl functionalized composition, applied in the eye while producing irritation. field, can solve the problems of high stimulation, high cost, eye irritation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0136] Preparation of Acetoacetyl Functional Latex Polymers
[0137] To the reactor was charged 775.2 parts deionized water and 18.4 parts RHODAPEX CO-436 (ie "CO-436"). The reaction mixture was heated to 75°C under a nitrogen blanket. A pre-emulsion was formed during heating which contained: 336.4 parts deionized water, 12.2 parts CO-436, 0.8 parts ammonium persulfate, 370.0 parts butyl acrylate, 190.2 parts methyl methacrylate, 118.3 parts benzene Ethylene, 78.9 parts AAEM, and 31.5 parts methacrylic acid. Once the reaction mixture reached 75°C, a 5% preemulsion was added to the reactor, followed by a mixture of 2.4 parts ammonium persulfate and 7.5 parts water. The reaction was held for 5-10 minutes, followed by an exotherm, then the remainder of the preemulsion was added to the reactor over 2 hours. During the polymerization reaction, the reaction temperature was maintained between 80°C and 85°C. Once the preemulsion feed was complete, the vessel was rinsed with 20 par...
Embodiment 2
[0139] Preparation of UV-curable acetoacetyl-functional coating compositions
[0140] With stirring, 100 grams of deionized water, 14.2 grams of RHODAPAN UB, and 200 grams of EOTMPTA were added to a stainless steel mixing vessel. Blend the mixture until a pre-emulsion forms.
[0141] With stirring, 1000 grams of the latex polymer from Example 1, 93 grams of deionized water and 157 grams of the EOTMPTA preemulsion prepared above were added to a stainless steel mixing vessel. The mixture was then kept under stirring for 8 hours until the EOTMPTA migrated into the latex polymer. 15 grams of IRGACURE 500 was then added to the mixture and kept under stirring for an additional 15 minutes. The mixture was then left overnight to allow any trapped air to escape.
[0142] The resulting mixture will cure to a hard, chemically resistant finish upon exposure to UV light. The mixture from Example 2 also cured to a hard, chemically resistant finish under electron beam radiation without p...
Embodiment 3
[0144] Preparation of (Meth)acrylate Functional Polyurethane Dispersions (PUD) Using TMPTA Reactive Diluent
[0145] Add 96.0 parts of TMPTA, 48.0 parts of 4-HBA, 91.4 parts of DESMOPHEN S-105-110 polyester diol, 29.3 parts of DMPA, 9.6 parts of TMP, 258.9 parts of isophorone diisocyanate and 500 ppm of 2,6-ditertiary Butyl-4-methylphenol. The reaction mixture was heated to 80°C under air sparge whereupon 250 ppm DBTDL was added and the reaction was carried out until the isocyanate content was below 9.2%. The polyurethane was cooled to 65°C and then neutralized with 22.1 parts of TEA. The polyurethane viscosity at 65°C was less than 6,000 centipoise (cps) as measured by a Brookfield DV-I+Viscometer (Viscometer) and #31 spindle at 1.5 revolutions per minute (RPM).
[0146] The (meth)acrylate polyurethane formed above was then dispersed into 895.5 parts of room temperature deionized water at a process temperature of 65°C, followed by chain extension with 51.1 parts of hydrazin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com