Production of cabergoline and novel polymorphic form thereof
A kind of technology of cabergoline and polymorph, applied in the field of producing cabergoline
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1: synthetic cabergoline
[0055] Add N-(3-dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride (EDAC.HCl) to a stirred mixture of trifluoromethylbenzene (BTF) and 25% w / w potassium carbonate solution middle. The mixture was stirred until a clear biphasic solution was obtained. The layers were separated and the lower liquid layer was discarded. The upper organic layer was stirred with anhydrous potassium carbonate and filtered to provide a solution of EDAC in BTF.
[0056] Stir the BTF suspension of the compound of formula (II) at 18°C to 24°C, and add the required amount of EDAC to the BTF solution. The resulting suspension was then heated to 35°C to 38°C and kept at this temperature until the reaction was complete. The solution was filtered and purified water was added. Glacial acetic acid was then added to bring the pH to 5.0 to 5.5. The upper liquid phase was separated. Methyl tert-butyl ether was added to the upper liquid phase, and 20% w / w po...
Embodiment 2
[0059] Example 2 Formation of TAME solvates
[0060] Cabergoline (4.0 g) was dissolved in 10 ml methyl tert-amyl ether and placed on a heating mantle set to 50°C. When the temperature reached 41°C, a clear solution was obtained after 15 minutes. The solution was filtered through a 0.45 micron filter and the resulting solution was cooled to 20°C to 26°C and seeded with 1% w / w pure Cabergoline Form I.
[0061] Crystallization started at 27°C and the resulting suspension was cooled to 0°C to 5°C and kept at this temperature for more than 16.5 hours. The resulting white solid was filtered under nitrogen atmosphere. The product obtained had a wet weight of 3.55 g, corresponding to a recovery of 88.8%.
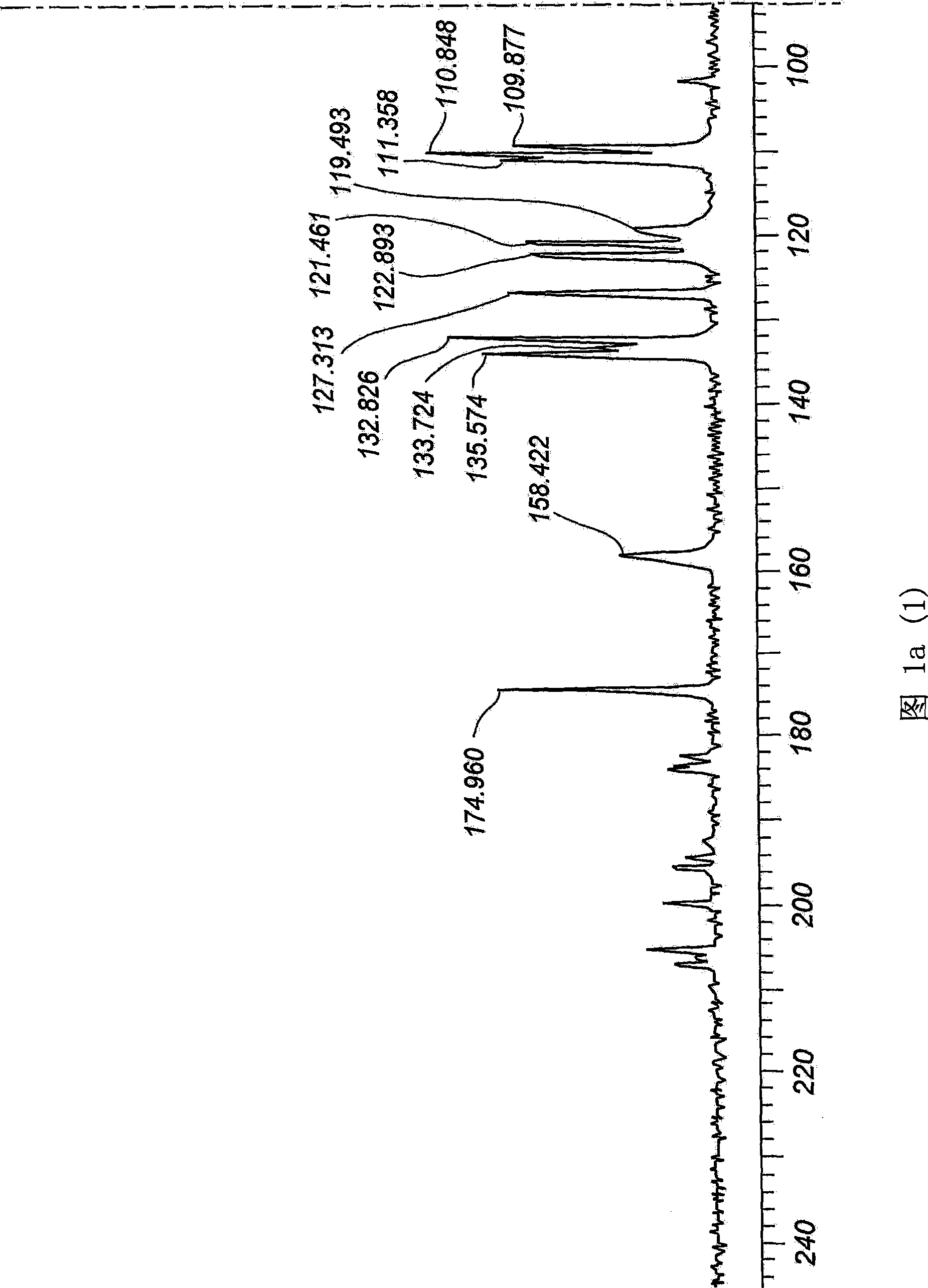
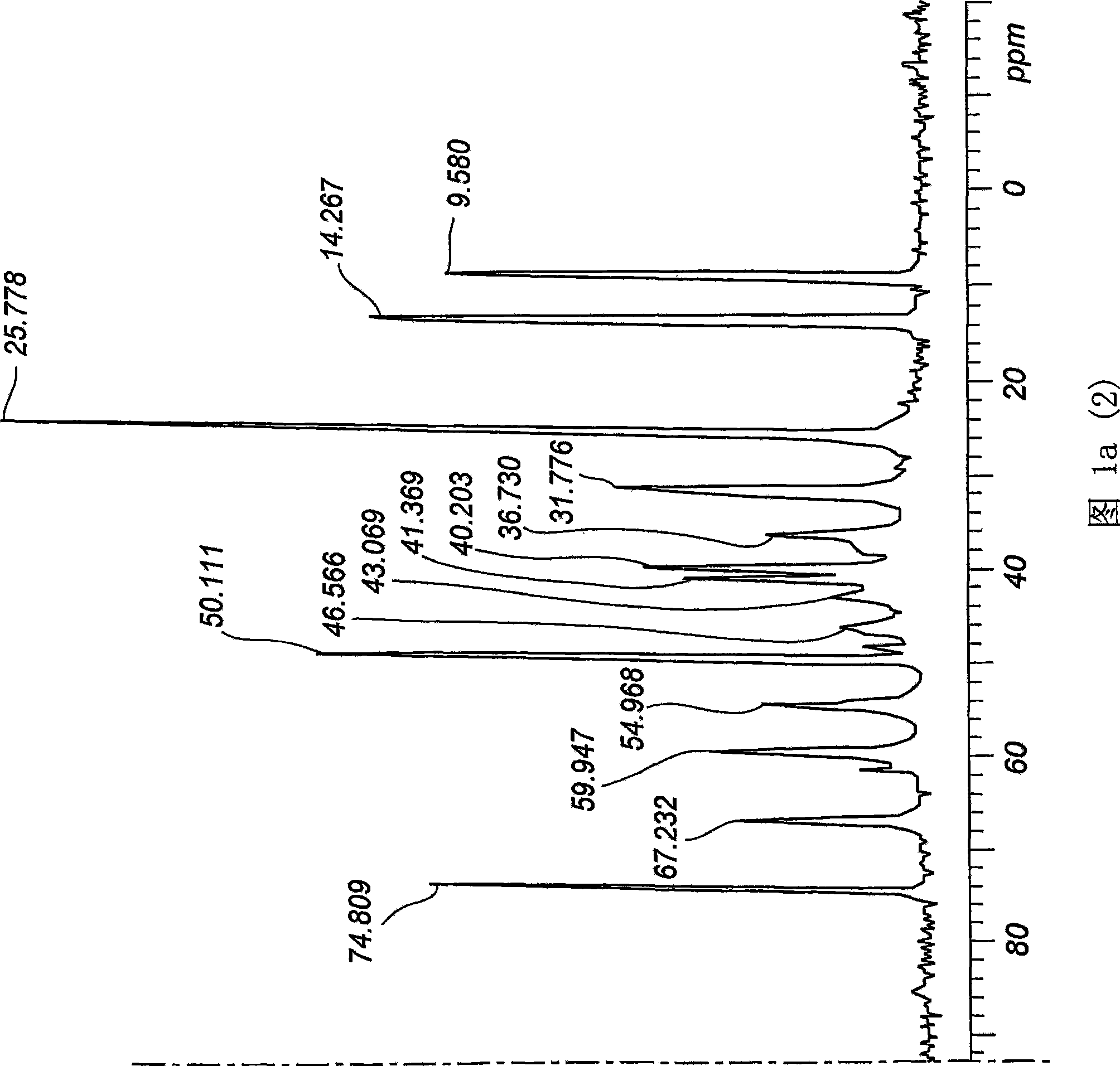
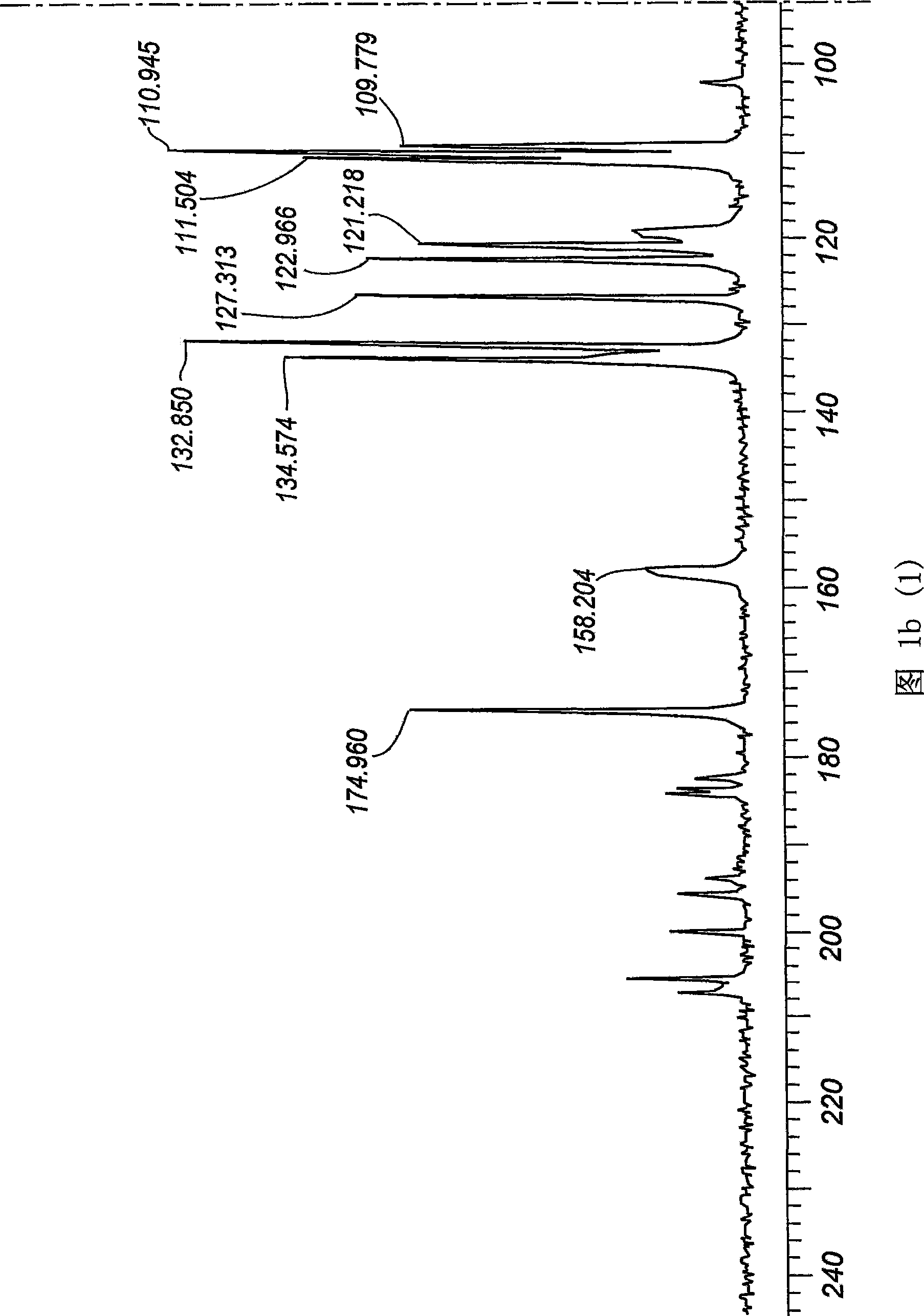
[0062] The product sample was subjected to 13C mass spectrometry, differential scanning calorimetry (DSC), DRIFT IR, DSC, X-ray crystallography, gas chromatography, HPLC and particle size analysis to determine whether it was a new crystal form. The product was also found to be o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com