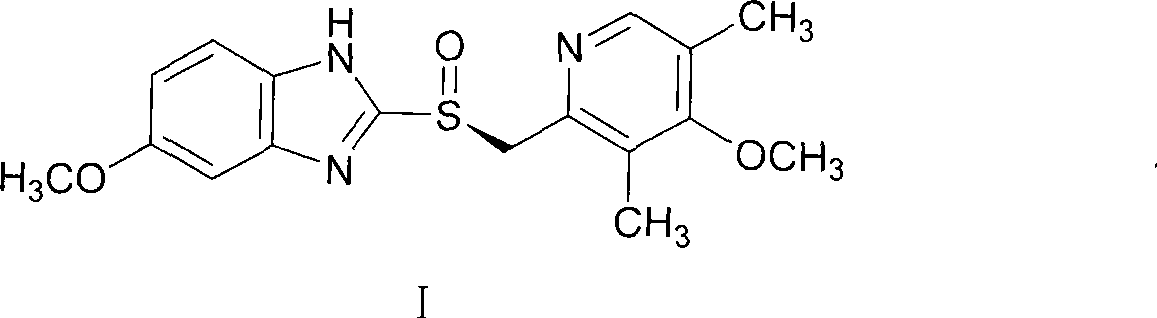
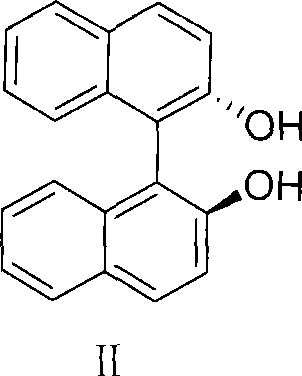
Method for preparing S-omeprazole and salt thereof by forming inclusion complex with (S)-(-)-1,1'-dinaphthalene-2,2'-diol
An inclusion complex, omeprazole technology, applied in the preparation of S-Omeprazole by forming an inclusion complex with (S)-(-)-1,1'-binaphthyl-2,2'-diphenol In the field of meprazole and its salts, it can solve the problems that it is not suitable for industrial production and large-scale production, and achieve the effects of high ee value, low environmental pollution and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046]Example 1: Preparation of S-omeprazole and (S)-(-)-1,1'-binaphthyl-2,2'-diphenol inclusion complex 249g (0.87mol) of (S)- (-)-1,1'-binaphthyl-2,2'-diphenol was dissolved in 3L of toluene, at 80°C, 300g (0.87mol) omeprazole was added to the solution, and cooled within 15 minutes after dissolution To 65°C, add 9g of S-omeprazole and (S)-(-)-1,1'-binaphthyl-2,2'-diphenol inclusion complex seed crystals, then drop to 45°C within 20min, A solid was precipitated, filtered, washed with toluene, and dried to obtain off-white crystals. (261.5g, yield 92%, ee value 98.5%)
Embodiment 2
[0047] Example 2: Preparation of S-omeprazole and (S)-(-)-1,1'-binaphthyl-2,2'-diphenol inclusion complex
[0048] 249g (0.87mol) of (S)-(-)-1,1'-binaphthyl-2,2'-diphenol was dissolved in 3L of toluene, and at 70°C, 300g (0.87mol) of azulene was added to the solution. For meprazole, cool to 60°C within 15 minutes after dissolving, add 9g of S-omeprazole and (S)-(-)-1,1'-binaphthyl-2,2'-diphenol inclusion complex crystal Seed, and then lowered to 40°C within 20 minutes, a solid precipitated, filtered, washed with toluene, and dried to obtain off-white crystals. (253g, yield 90%, ee value 98.2%)
Embodiment 3
[0049] Embodiment 3: the preparation of S-omeprazole magnesium salt
[0050] Dissolve 48.6g (0.87mol) of potassium hydroxide in 1.5L of water to form a potassium hydroxide solution, then add 300g (0.485mol) of S-omeprazole and (S)-(-)-1,1' - Binaphthalene-2,2'-diphenol inclusion complex (prepared by the method of Example 1 or 2), add 1.3L of isopropyl acetate, separate the water layer, and then extract the water layer with isopropyl acetate until Remove (S)-(-)-1,1'-binaphthyl-2,2'-diphenol in the water layer, and add a pre-prepared magnesium chloride aqueous solution (magnesium chloride hexahydrate 93.4g, water 240g) into the water layer , formed a precipitate, was filtered, and the solid was washed with water to obtain 202.6 g of a wet product (the yield on a dry basis was 92%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com