Uses of 2,5-dewatering-D-glucitol and the like in preparing cancer treatment medicament
A technology of glucitol and gluconic acid is applied in the field of preparing medicines for the treatment of cancer, and can solve problems such as cell damage and adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
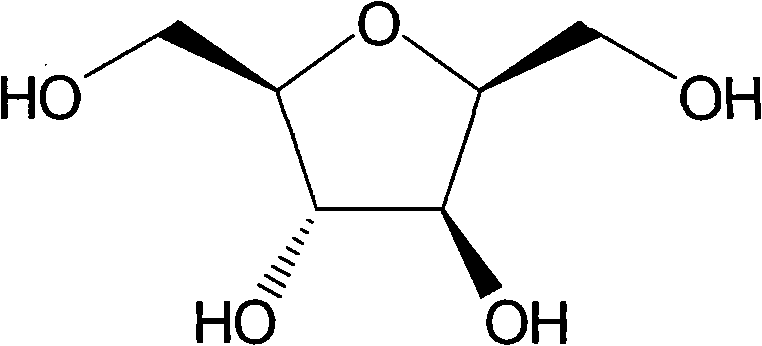
[0031] Example 1 Preparation of 2,5-anhydro-D-glucitol
[0032] Dissolve 5.0 grams of 2,5-anhydro-4,5-diacetyl-D-glucitol (for preparation, refer to Azeez M. Mubarak and Daniel M. Brown. J.C.S. Perkin I. 1982, 809-813) in 50 mL of methanol Add 1molL dropwise under ice-water bath -1 10 mL of sodium methoxide in methanol. After stirring at room temperature for 24 hours, it was adjusted to neutrality with a strong cationic resin. The resin was filtered off, washed three times with methanol, the filtrates were combined, concentrated under reduced pressure, and subjected to flash silica gel column chromatography (dichloromethane:methanol=10:1) to obtain 1.7 grams of 2,5-anhydro-D-glucitol, yield: 84.2 %. Its H NMR spectrum is consistent with that reported in the literature (J.Org.Chem.2000, 65, 5632-5638).
Embodiment 2
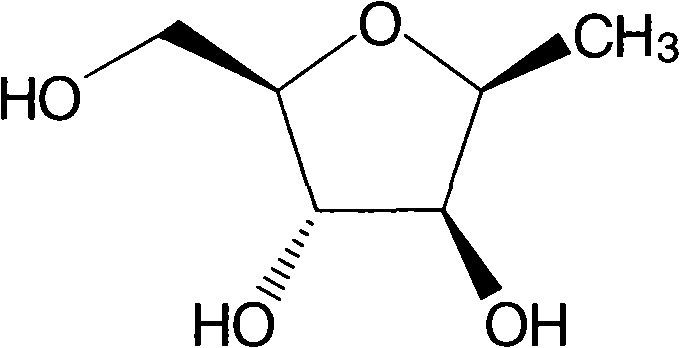
[0033] Example 2 Preparation of 2,5-anhydro-1-deoxy-D-glucitol
[0034] 5.0 grams of 2,5-anhydro-1-p-toluenesulfonyl-4,5-diacetyl-D-glucitol (preparation can refer to Azeez M.Mubarak and Daniel M.Brown.J.C.S.Perkin I.1982,809-813 ) was dissolved in 30 mL of DMSO, and then 1.4 g of sodium borohydride was added. Stir at about 70°C for 6 hours, then cool, pour the reaction solution into 150mL ice water, extract with ether (40mL×4), wash with saturated brine, anhydrous Na 2 SO 4 Drying, concentration under reduced pressure, and flash silica gel column chromatography (petroleum ether: ethyl acetate = 5:1) yielded 2.5 g of 2,5-anhydro-1-deoxy-4,5-diacetyl-D-glucitol, Yield: 73.5%. 1 H NMR (300MHz, CDCl 3 )δppm: 1.37-1.39 (d, 3H), 2.70 (s, 1H), 4.21-4.30 (m, 3H), 4.59-4.66 (m, 3H), 5.21-5.22 (m, 3H), 7.40-8.07 ( m, 10H); ESI MS m / z: 379.1 [M+Na] + .
[0035] Dissolve 2.5 grams of 2,5-anhydro-1-deoxy-4,5-diacetyl-D-glucitol from the previous step in 25 mL of methanol, and add 1...
Embodiment 3
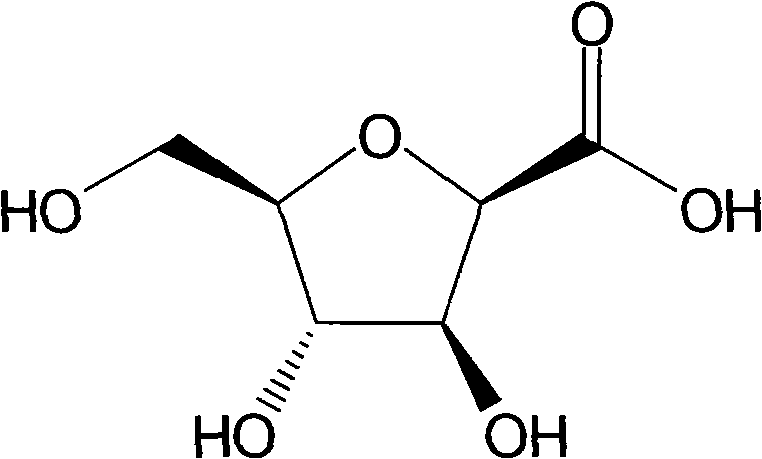
[0036] Example 3 Preparation of 2,5-anhydro-D-gluconic acid
[0037] Dissolve 5 g of 2,5-anhydro-3,4,5-tribenzyl-D-glucitol (refer to Carbohydrate Research, 171, 1-11; 1987) in 30 mL of DMF, and add 15 grams of pyridinium dichromate (PDC). After stirring vigorously at room temperature for 24 hours, the reaction solution was poured into 150 mL of ice water, extracted with ethyl acetate (40 mL×4), washed with saturated brine, anhydrous Na 2 SO 4 Drying, evaporation to dryness, and flash silica gel column chromatography (petroleum ether: ethyl acetate = 2:1) gave 3.8 g of 2,5-anhydro-3,4,5-tribenzyl-D-gluconic acid, yield: 73.6%.
[0038] Dissolve 3.8 grams of 2,5-anhydro-3,4,5-tribenzyl-D-gluconic acid from the previous step in 50 mL of ethanol, add 0.5 grams of 10% palladium carbon, and hydrogenate at room temperature and pressure for 48 hours. After the reaction was complete, the palladium carbon was removed by filtration, and the filtrate was evaporated to dryness. Silica...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com