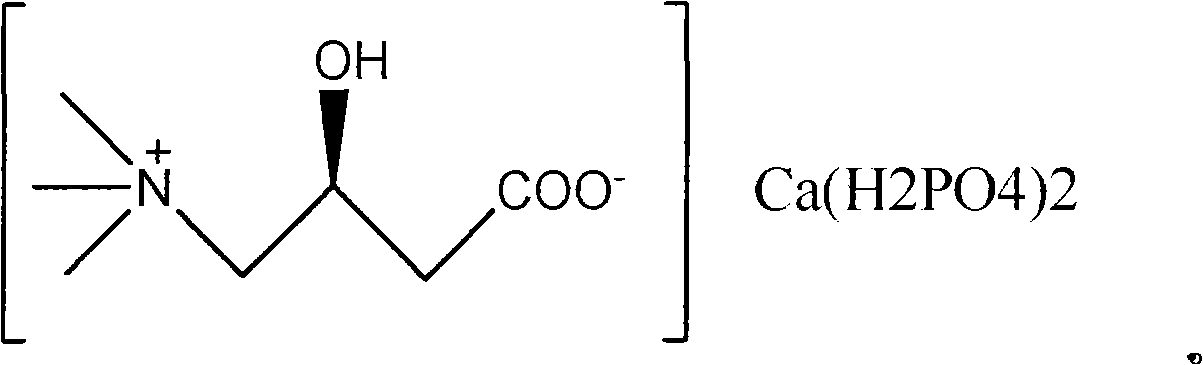
Levorotation carnitine acid calcium phosphate, preparation method and use thereof
A technology of L-carnitine calcium dihydrogen phosphate and its use, applied in the preparation of pharmaceutical intermediates and food additives, nutritional supplement compositions for calcium and phosphorus supplementation, L-carnitine calcium dihydrogen phosphate and its preparation fields, can solve the problem of There are no problems such as mass production and expensive prices, and it is suitable for oral administration, increasing the effect of calcium and phosphorus supplementation, strong and more functional nutrition and therapeutic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of L-Carnitine Dicalcium Phosphate
[0028]
[0029] Dissolve calcium hydroxide in water, add phosphoric acid, stir and react for 2-8 hours, add L-carnitine after filtration, stir at room temperature for 8-10 hours, concentrate, and crystallize in ethanol to obtain L-carnitine dicalcium phosphate. Yield 98.6%.
[0030] The obtained L-carnitine calcium dihydrogen phosphate has good fluidity, and the powder is uniform, and more than 98% of the powder is less than 40 mesh. Good water solubility under acidic conditions. Expose for 24 hours at a relative humidity of 60±5% at 25°C, no agglomeration and stickiness, good moisture absorption resistance, and a calcium content of 9.28%. Moisture <1%.
[0031] [α] D 20 =-10.08 (1%H 2 O)
[0032] HPLC:
[0033] Column: APS-2HYPERSIL (NH 2 )(5μm)250×4.6mm
[0034] Temperature: 30°C
[0035] Mobile phase: KH 2 PO 4 / Acetonitrile(70 / 30)(v / v)
[0036] pH: 4.0H 3 PO 4
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com