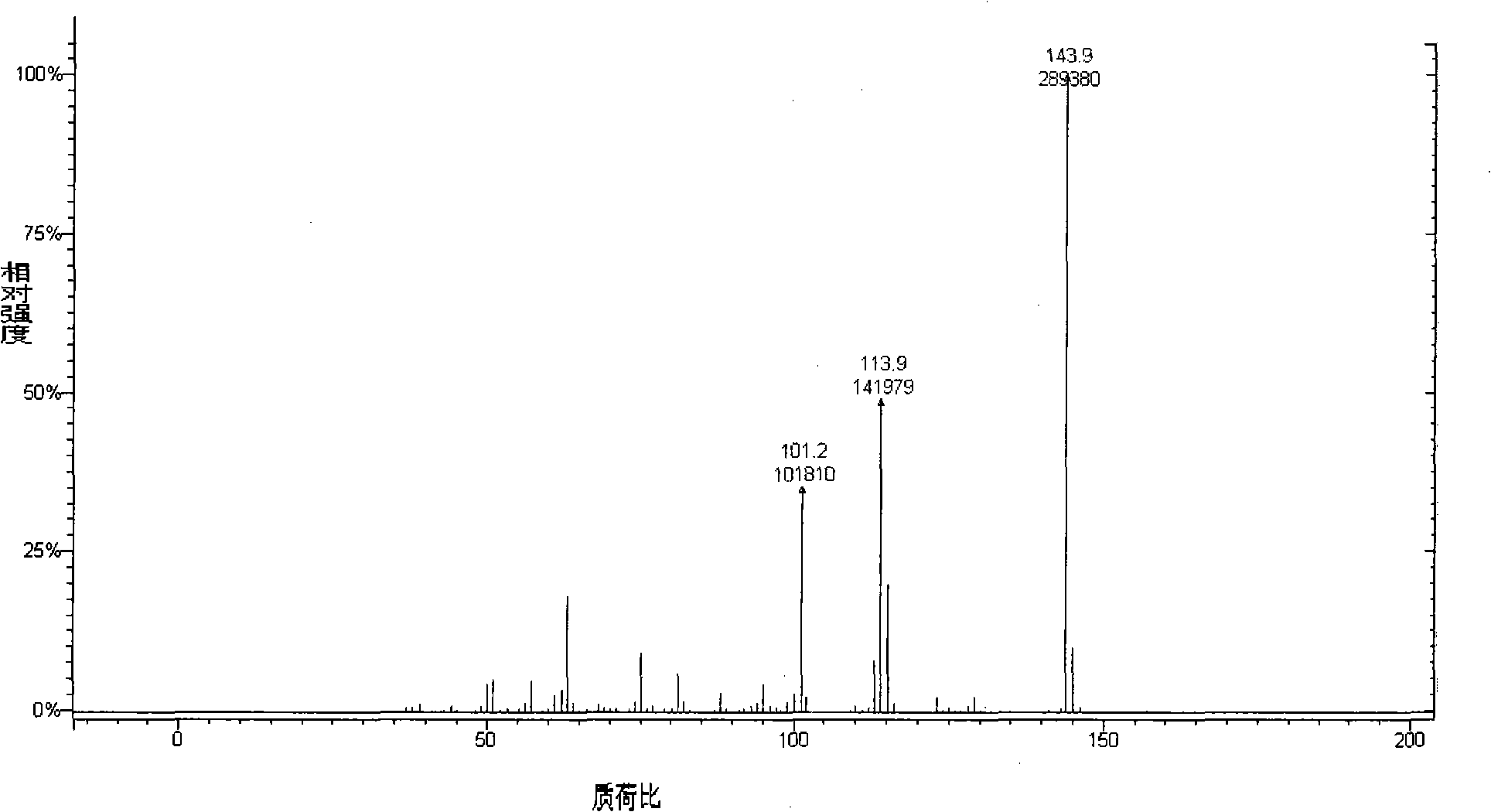
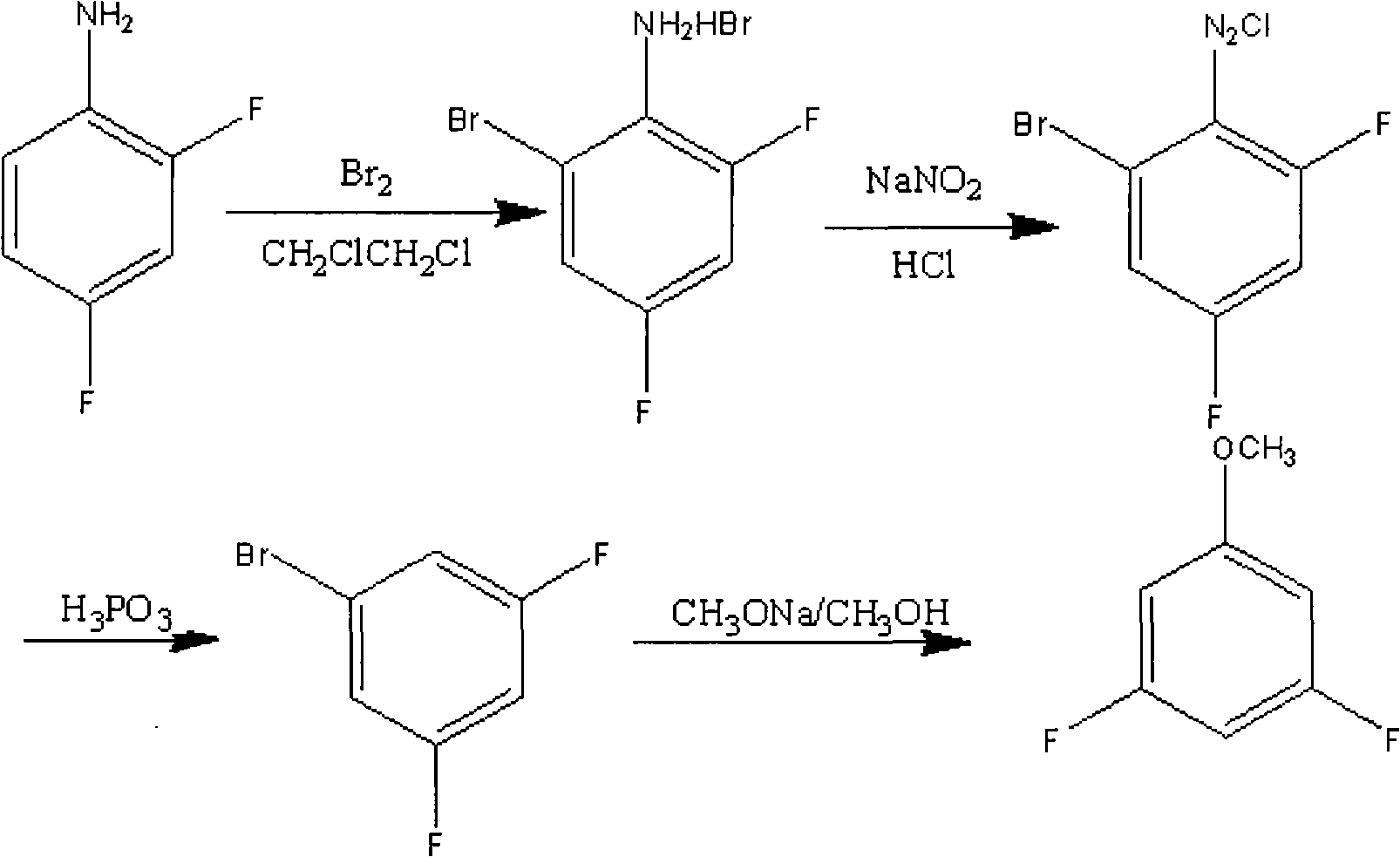
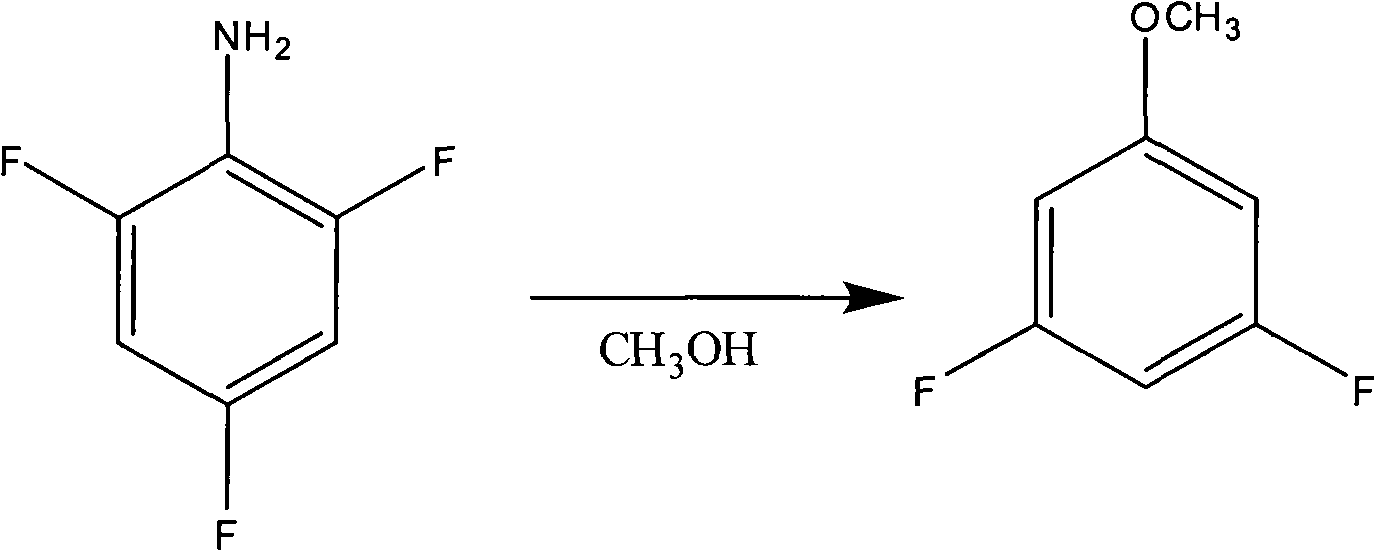
Preparation method of 3,5-difluoroanisole
A technology of difluoroanisole and trifluorobenzene, which is applied in the field of preparation of organic compounds, can solve the problems of long reaction time, low yield, and only 68% yield, and achieve the effect of high product yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] In a 5L three-necked flask equipped with a thermometer, a spherical condenser and a mechanical stirrer, add a solution containing 10.72mol (578.8g) of sodium methoxide at room temperature, the concentration of the sodium methoxide solution is 20% in terms of methanol, and then 938mL of solvent Methanol was slowly added into the three-necked flask, and stirred evenly at a slow speed.
[0056] Under the condition of stirring, 5.36mol (708g) of 1,3,5-trifluorobenzene was added dropwise into the reaction flask, and heated to raise the temperature, so that the reaction was carried out at 70-80°C. After the reaction was carried out for 5 hours, Heating was stopped, and the reaction product was allowed to cool naturally.
[0057] The cooled reaction product is carried out aftertreatment, and described aftertreatment comprises
[0058] 1) The reaction product is subjected to suction filtration to obtain a solid and a filtrate, and the solid is washed twice with methanol; after...
Embodiment 2
[0063] In a 5L three-necked flask equipped with a thermometer, a spherical condenser and a mechanical stirrer, add a solution containing 5.36mol (289.44g) of sodium methoxide at room temperature. The concentration of the sodium methoxide solution is 30% in terms of methanol, and then 1000mL of solvent Slowly add toluene into the three-necked flask, and stir evenly at a slow speed.
[0064] Under the condition of stirring, 5.36mol (708g) of 1,3,5-trifluorobenzene was added dropwise into the reaction flask, and heated to raise the temperature, so that the reaction was carried out at 60-80°C. After the reaction was carried out for 10 hours, Heating was stopped, and the reaction product was allowed to cool naturally.
[0065] The cooled reaction product is carried out aftertreatment, and described aftertreatment comprises
[0066] 1) The reaction product is subjected to suction filtration to obtain a solid and a filtrate, and the solid is washed twice with methanol; after the fil...
Embodiment 3
[0071] In a 5L three-neck flask equipped with a thermometer, a spherical condenser and a mechanical stirrer, add a solution containing 8.04mol (434.16g) of sodium methoxide at room temperature. The concentration of the sodium methoxide solution is 40% in terms of methanol, and then 950mL of solvent Dimethylformamide was slowly added into the three-necked flask, and stirred evenly at a slow speed.
[0072] Under the condition of stirring, 5.36mol (708g) of 1,3,5-trifluorobenzene was added dropwise into the reaction flask, and heated to raise the temperature, so that the reaction was carried out at 60-100°C. After the reaction was carried out for 18 hours, Heating was stopped, and the reaction product was allowed to cool naturally.
[0073] The cooled reaction product is carried out aftertreatment, and described aftertreatment comprises
[0074] 1) The reaction product is subjected to suction filtration to obtain a solid and a filtrate, and the solid is washed twice with methan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com