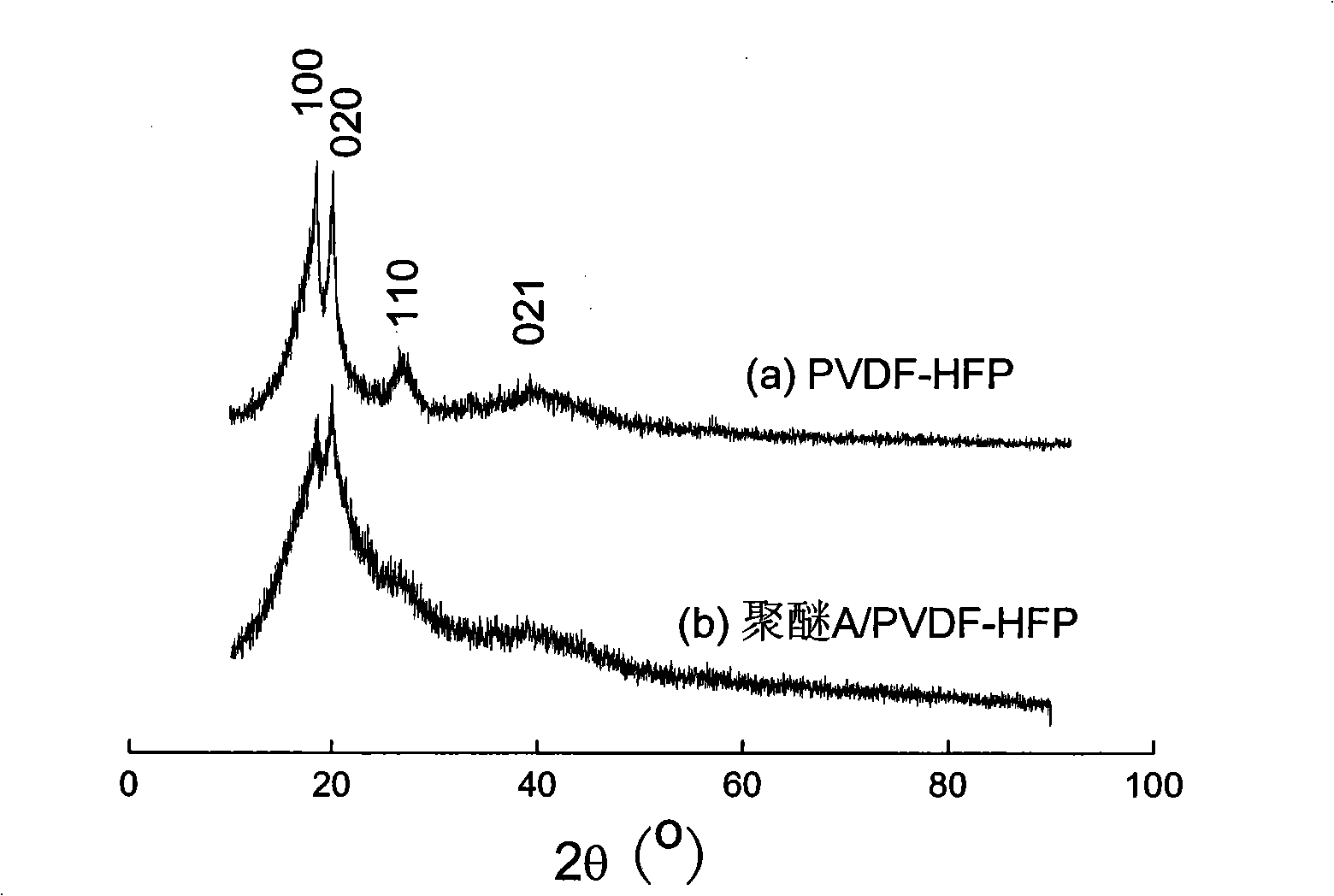
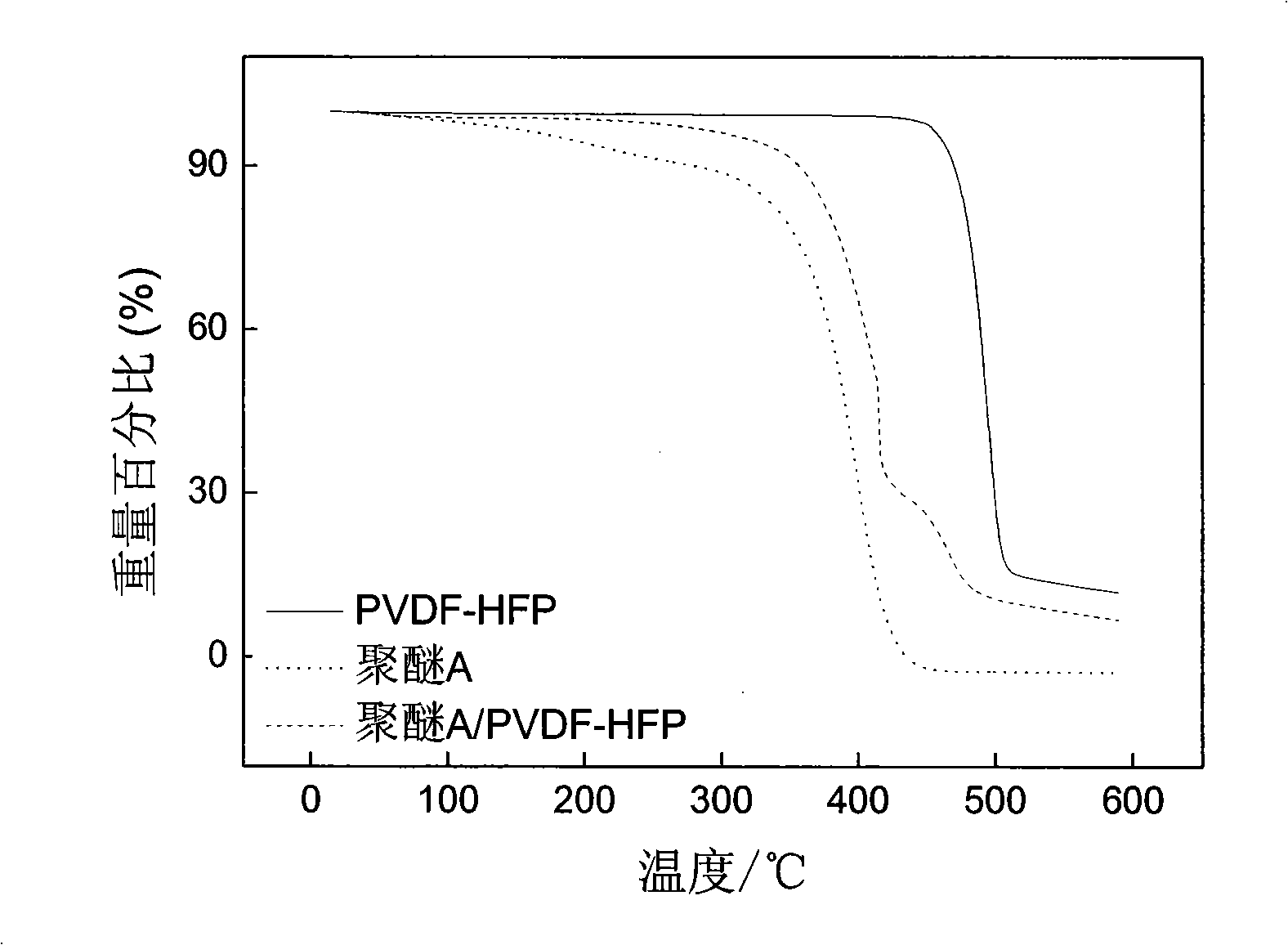
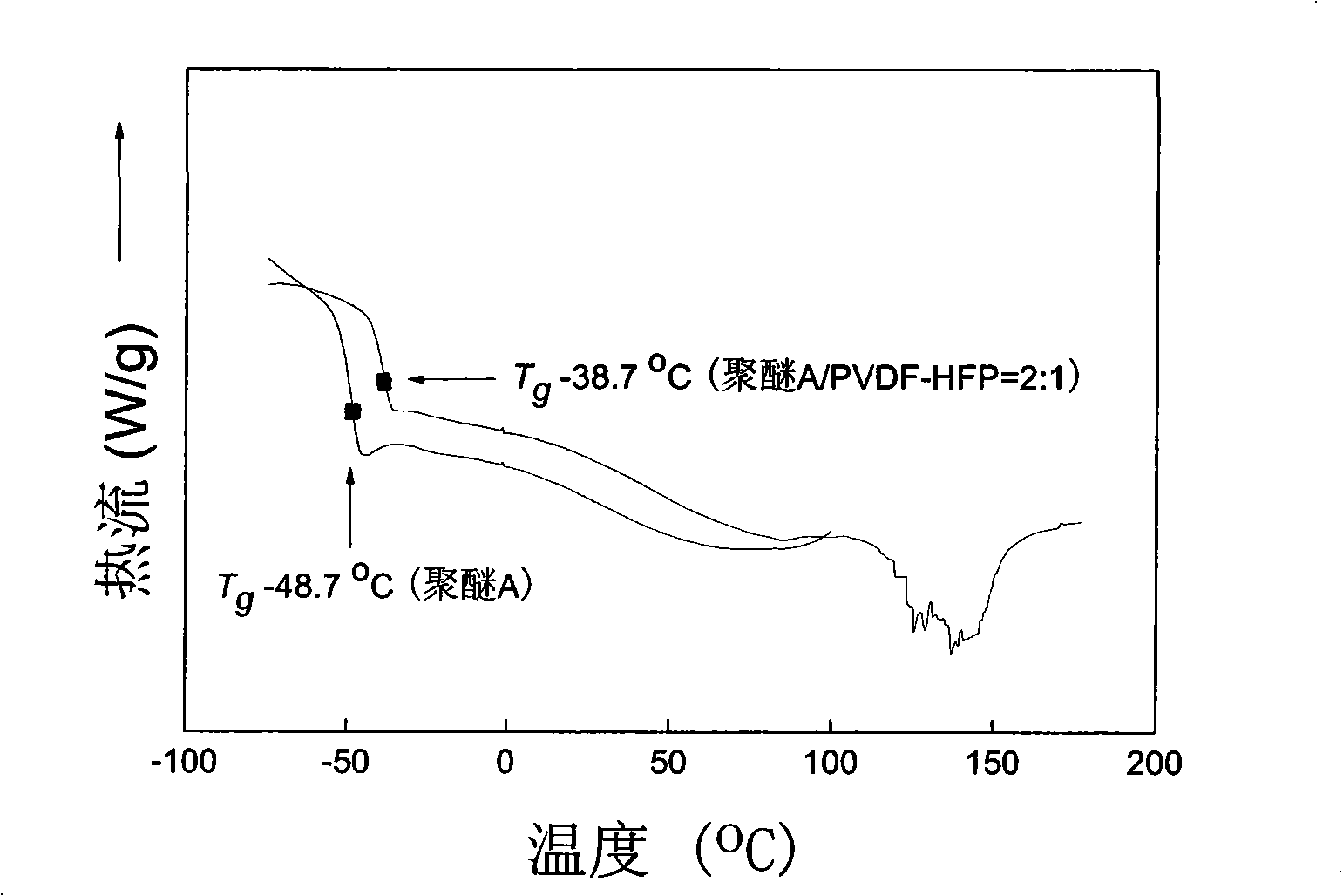
Hyperbranched polyether type solid polymer elecrolytes and preparation thereof
A technology of hyperbranched polyether and solid polymer, applied in the direction of circuits, electrical components, secondary batteries, etc., to achieve the effect of lowering the glass transition temperature, good mechanical properties, and good mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The polymer electrolyte matrix material A used in Example 1 is based on the ion-conducting group monomer structure 3-(2-hydroxytriethoxy)-3'-methyloxetane as a repeating unit (with the following structure ):
[0027]
[0028] with BF 3 Diethyl ether is used as an initiator to polymerize a class of hyperbranched polyether A without benzene rings, and its weight average molecular weight is 10000; the polymer electrolyte matrix material B used is poly(vinylidene fluoride-hexafluoropropylene) for short: PVDF-HFP; Lithium salt C is lithium bis(trifluoromethylsulfonyl)imide, abbreviated as LiTFSI. All raw materials were vacuum dried before use. Take a certain amount of hyperbranched polyether (abbreviated as polyether A) and poly(vinylidene fluoride-hexafluoropropylene) (PVDF-HFP) according to the mass ratio of 2:1, dissolve them in tetrahydrofuran, stir until uniform, and then Add LiTFSI with a mass percentage of 20% (the mass percentage is calculated based on the tota...
Embodiment 2
[0030] Prepare the polymer electrolyte in the same manner as in Example 1, the difference is that the polymer matrix material B is a PET material with better mechanical properties, and the hyperbranched polyether (polyether A) and polyethylene terephthalate Glycol esters (PET) take a certain amount according to the mass ratio of 1:3, dissolve with tetrahydrofuran, stir until uniform, then add different mass percentages of LiTFSI (mass percentages are calculated according to the total mass of the polymer electrolyte membrane), and continue to stir until the lithium salt is evenly distributed in the solution. The solution was poured into a polytetrafluoroethylene film tool, the solvent was evaporated, and then dried in a vacuum oven at 50° C. for 18 h.
[0031] The prepared solid polymer electrolyte (SPE) was sandwiched between two stainless steel (SS) electrodes to form a SS / SPE / SS battery, and the ionic conductivity of the prepared sample was tested for ionic conductivity ( ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com