Method for synthesizing (R)-9-(2-phosphate methoxy propyl)adenine
A technology of methoxypropyl phosphate and a synthesis method, which is applied in the field of synthesis of -9-adenine, can solve the problems of low yield, high price and high cost of trimethylchlorosilane, and achieves no environmental pollution and low price. , the effect of high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
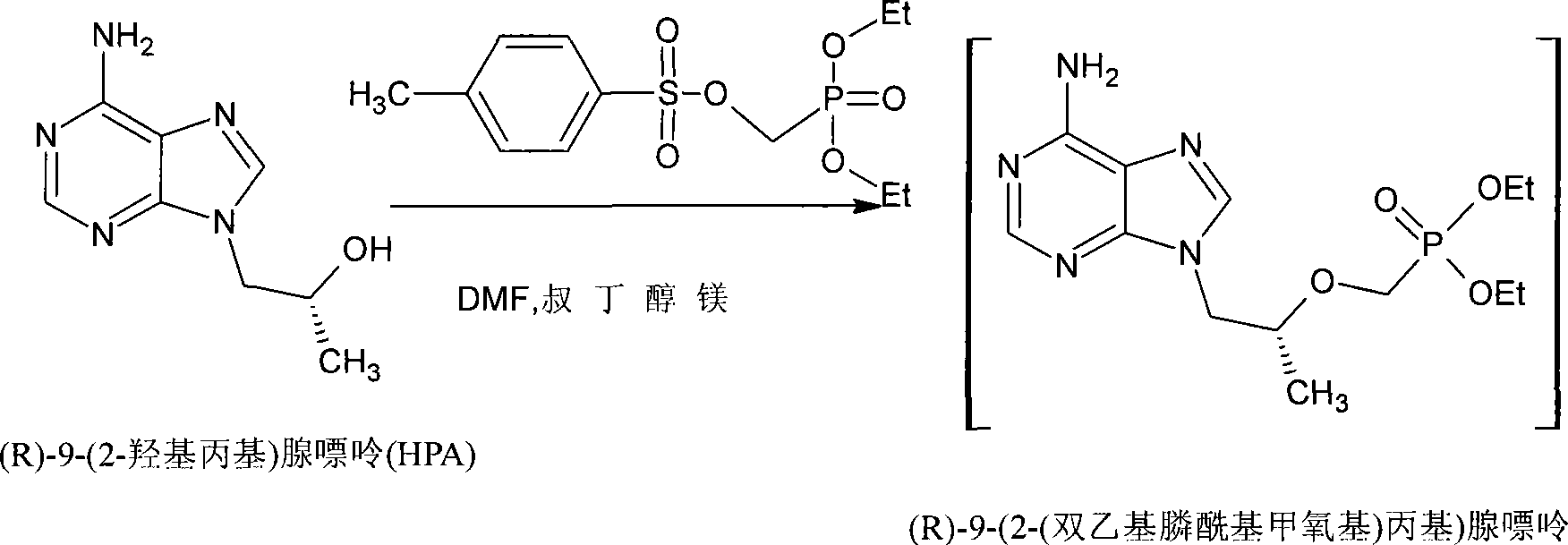
[0019] Synthesis of (R)-9-(2-hydroxypropyl)adenine (HPA)
[0020] Adenine (10.4g, 76.6mmol), sodium hydroxide (0.12g, 3mmol), (R)-1,2-propene carbonate (8.6g, 84.2mmol), DMF (72g) was heated to 132 to 138°C , keep the reaction until the cloudy liquid dissolves and then keep it warm for 1 hour. Cool to below 100°C, adjust PH≈7 with methanesulfonic acid. Cool to below 80°C, add 150ml of alcohol, stir and cool to below 10°C and keep warm for 3 hours, filter with suction, and dry to obtain 11g white powder with HPLC purity of 90-100% (yield is 74% in terms of adenine).
Embodiment 2
[0022] Synthesis of (R)-9-(2-(bisethylphosphono)methoxypropyl)adenine (PMPA diester) HPA (100 g, 0.518 mol) was dissolved in DMF (200 ml) at room temperature, Add magnesium tert-butoxide (71g, 0.415mol), heat to 60°C and keep it warm for 1 hour, raise the temperature to above 74°C, add diethyl-p-toluenesulfonate methylphosphonate (200g, 0.6216mol) dropwise in about 2 hours, Then keep it warm at 74-78°C for 5 hours. Cool to room temperature, add glacial acetic acid (60 g, 1.0 mol), stir for 15 minutes, and concentrate the solution under reduced pressure to obtain PMPA diester as a viscous liquid.
Embodiment 3
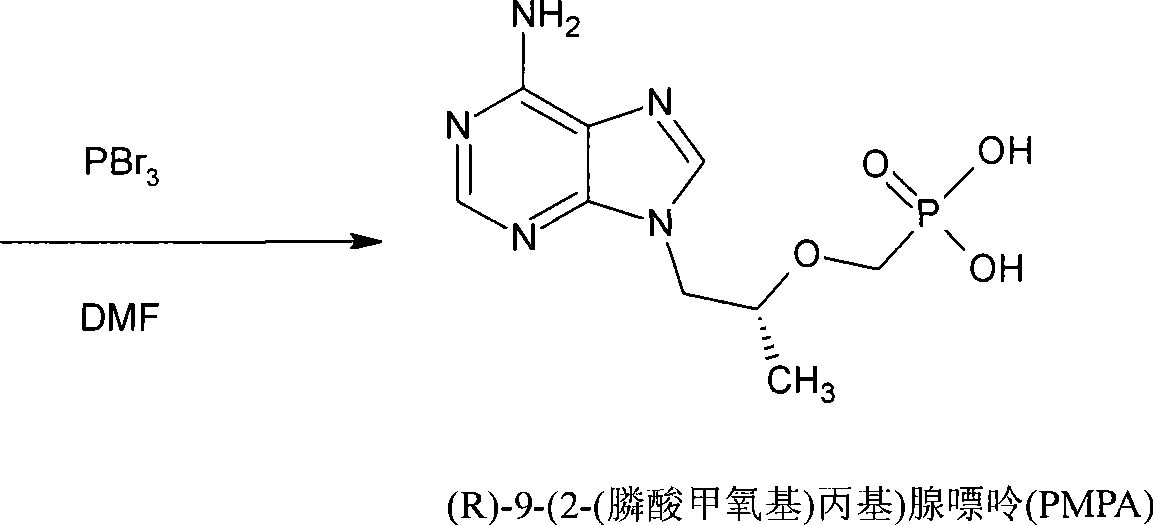
[0024] Synthesis of (R)-9-(2-(methoxypropyl phosphate)adenine (PMPA)
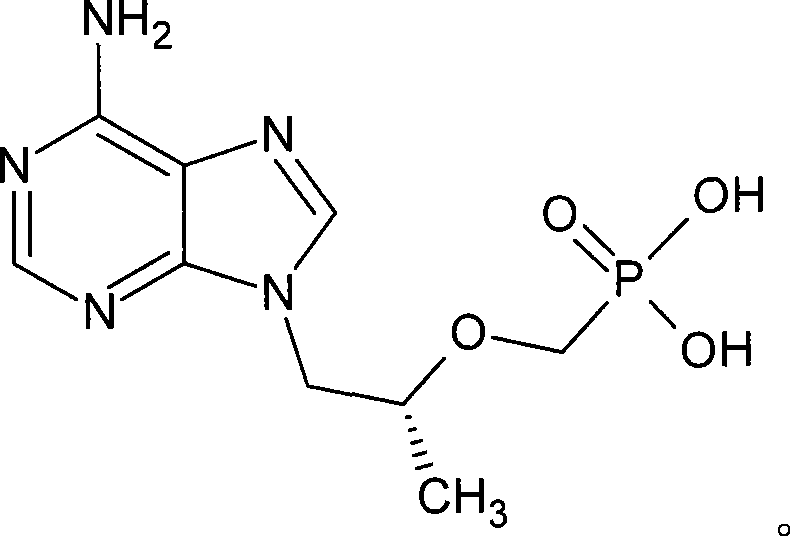
[0025] The viscous liquid obtained in Example 2 was added with 200ml DMF, cooled to below 0°C, and phosphorus tribromide (112g, 0.414mol) was added dropwise while keeping the temperature not exceeding 50°C, and the temperature was raised to 70°C for 5 hours after dropping. After the reaction is complete, cool to below 0°C, add 40 g of water dropwise, and then concentrate the reaction solution under reduced pressure. Add 300ml of water to the concentrated solution, wash twice with 150ml of dichloromethane, adjust the pH of the aqueous layer to ≈3 with 1N sodium hydroxide solution, cool to below 10°C, keep warm for 3 hours, and filter with suction. The obtained PMPA crude product is refined with water to obtain a finished product, and the HPLC purity is 98-100%. PMPA dry product 70g (yield is 47% based on HPA).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com