Potassium borofluoride preparation method based on fluosilicic acid and boron rock
A technology of potassium fluoroborate and fluorosilicic acid is applied in the field of preparation of inorganic fluorides, which can solve the problems of high production cost, high price, shortage of borax boric acid supply, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
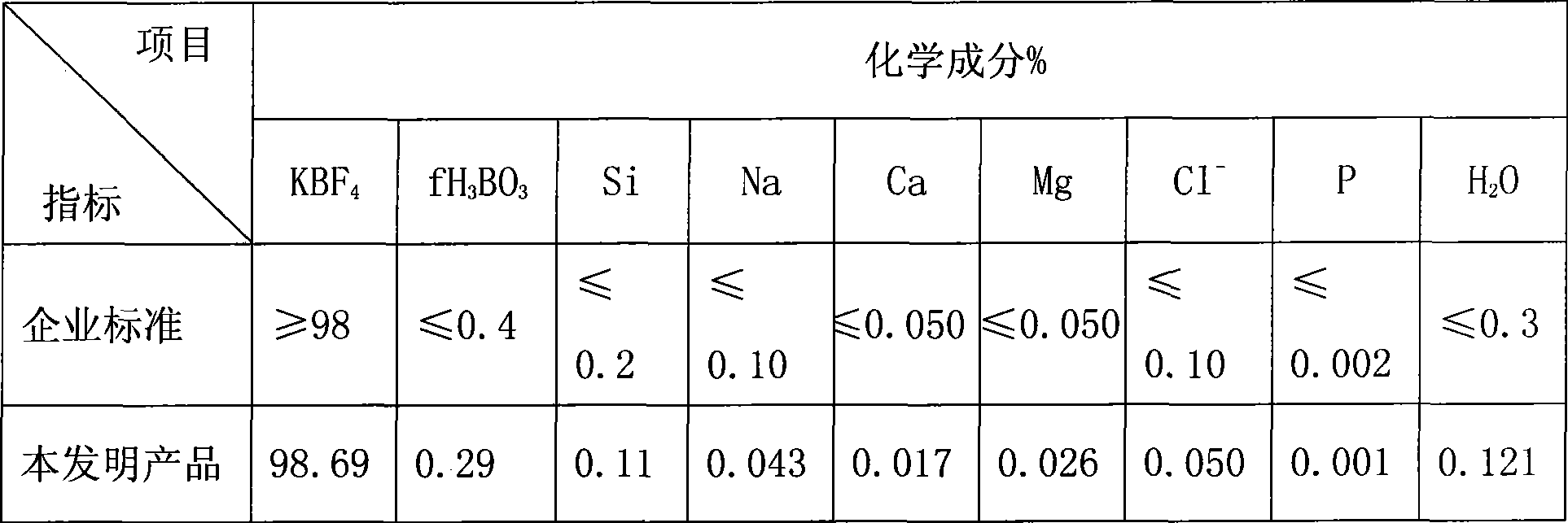
[0025] Embodiment 1: add fluorosilicic acid 6.2m in leaching tank 3 , the concentration is 10%, boron ore (B 2 o 3 15%) press B 2 o 3 Add in molar ratio of 1:0.8 to fluosilicic acid; leach at 95°C for 180 minutes, filter, add 300kg of N-type impurity remover to the filtrate to remove impurities for 60 minutes, and filter to obtain refined liquid, which is mixed with 2.5m 3 Potassium chloride solution (200g / l) was synthesized for 80 minutes at a reaction temperature of 70°C, then filtered, washed, separated, and dried to obtain 385kg of potassium fluoroborate product.
Embodiment 2
[0026] Embodiment 2: add fluosilicic acid 6.2m in leaching tank 3 , the concentration is 20%, boron ore (B 2 o 3 10%) press B 2 o 3 The molar ratio with fluosilicic acid is 1:1.2; leaching at 85°C for 140 minutes, filtering, adding 620kg of N-type impurity remover to the filtrate to remove impurities for 120 minutes, and filtering to obtain a refined solution, which is the same as 4.8m 3 Potassium chloride solution (220g / l) was synthesized for 90 minutes at a reaction temperature of 40°C, then filtered, washed, separated, and dried to obtain 950kg of potassium fluoroborate product.
Embodiment 3
[0027] Embodiment 3: add fluorosilicic acid 6.2m in leaching tank 3 , the concentration is 15%, boron ore (B 2 o 3 40%) press B 2 o 3 The molar ratio to fluosilicic acid is 1:0.6; leaching at 60°C for 300 minutes, filtering, adding 500kg of N-type impurity remover to the filtrate to remove impurities for 180 minutes, and filtering to obtain a refined solution, which is the same as 3.0m 3 Potassium chloride solution (250g / l) was synthesized for 150 minutes at a reaction temperature of 85°C, then filtered, washed, separated, and dried to obtain 770kg of potassium fluoroborate product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com