Absorbent charcoal based catalyst carrier, catalyst, preparation and uses thereof
A technology of catalyst carrier and activated carbon, which is applied in the direction of catalyst carrier, chemical instruments and methods, physical/chemical process catalysts, etc., and can solve the problems of serious desorption of metal ions and low acid strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
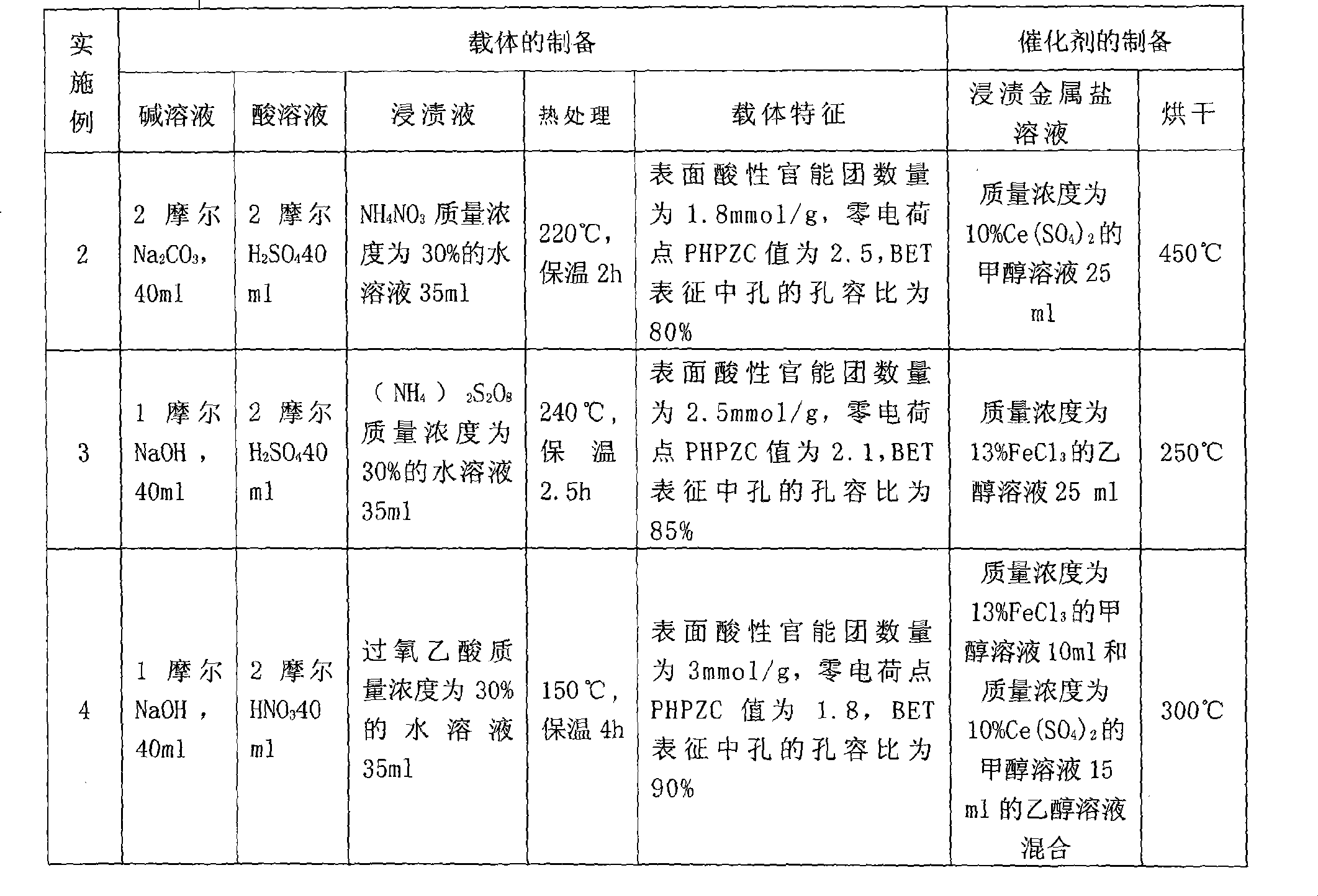
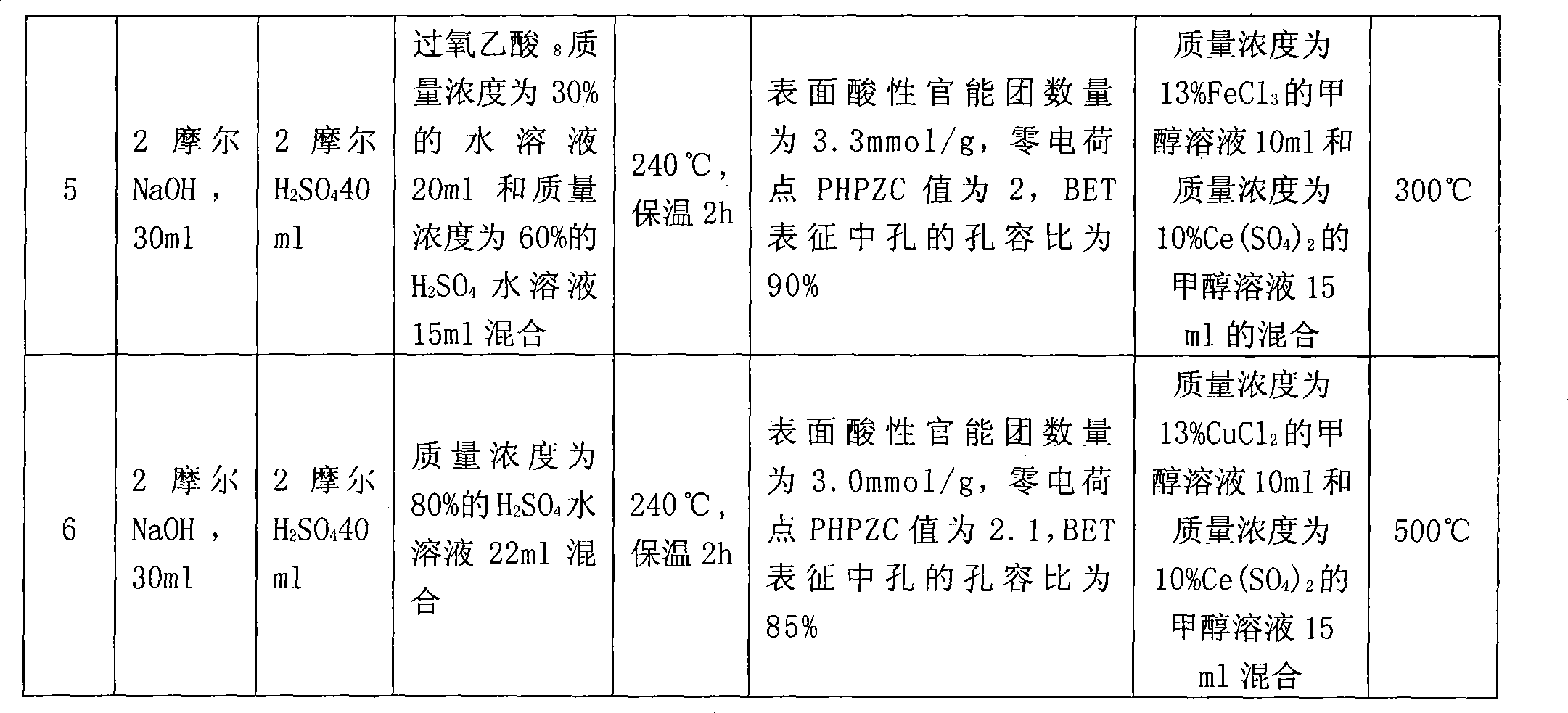
[0021] Example 1: Preparation method of activated carbon-based catalyst and its catalytic performance
[0022] A, the preparation method of activated carbon-based catalyst carrier, its process steps are:
[0023] a. Deashing treatment: add 10 grams of activated carbon with a particle size of 10 meshes (coal-based activated carbon with 10.8% ash content) into 30 ml of caustic soda solution with a concentration of 1.5 moles, slowly stir at 40-60 ° C for 1.5 hours, filter, wash with water to neutrality; then add 30 ml of hydrochloric acid solution with a concentration of 1.5 molar, slowly stir at 60-70 ° C for 2 hours, filter, and obtain deashed activated carbon for later use. It was detected that the ash content dropped to about 5%.
[0024] b. Impregnation pretreatment: Then take 25ml of sulfuric acid aqueous solution with a mass concentration of 60% and add it to the above deashing activated carbon for mixing, soak at room temperature for 12 hours, then filter and dry to obta...
Embodiment 7
[0038] According to the catalyst obtained in Examples 1-6, the catalytic activity experiment was carried out. The results showed that the catalyst could catalyze the synthesis of cyclohexanone-1,2-propanediol ketal and cyclopentanone-ethylene glycol ketal with high yield. above 88%. Moreover, the metal ions were not desorbed during the experiment, the amount of catalyst used was small, and the yield could be reused 6 times with little decrease. Its catalytic performance is significantly higher than that reported in the literature, and also higher than the catalyst carrier itself.
[0039] An example is as follows: The optimal synthesis reaction conditions are determined by orthogonal experiments: 0.1 mol of cyclopentanone, 1.70 mol of ethylene glycol, 9.5% (mass fraction) of the amount of cyclopentanone with water agent cyclohexane, catalyst When it is 9.5% (mass fraction) of the amount of cyclopentanone, it is added to a 100 mL three-necked flask, a water separator, a reflux...
Embodiment 8
[0041] Catalytic activity experiments were carried out according to the catalysts obtained in Examples 1-6. The results showed that the above catalysts could also catalyze the synthesis of esters such as dibutyl oxalate, tripentyl citrate and isoamyl acetate. The conversion rate remains above 92%. The esterification rate remained stable after repeated use for 6 times.
[0042] It shows that the prepared activated carbon-based catalyst is a good solid acid catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com