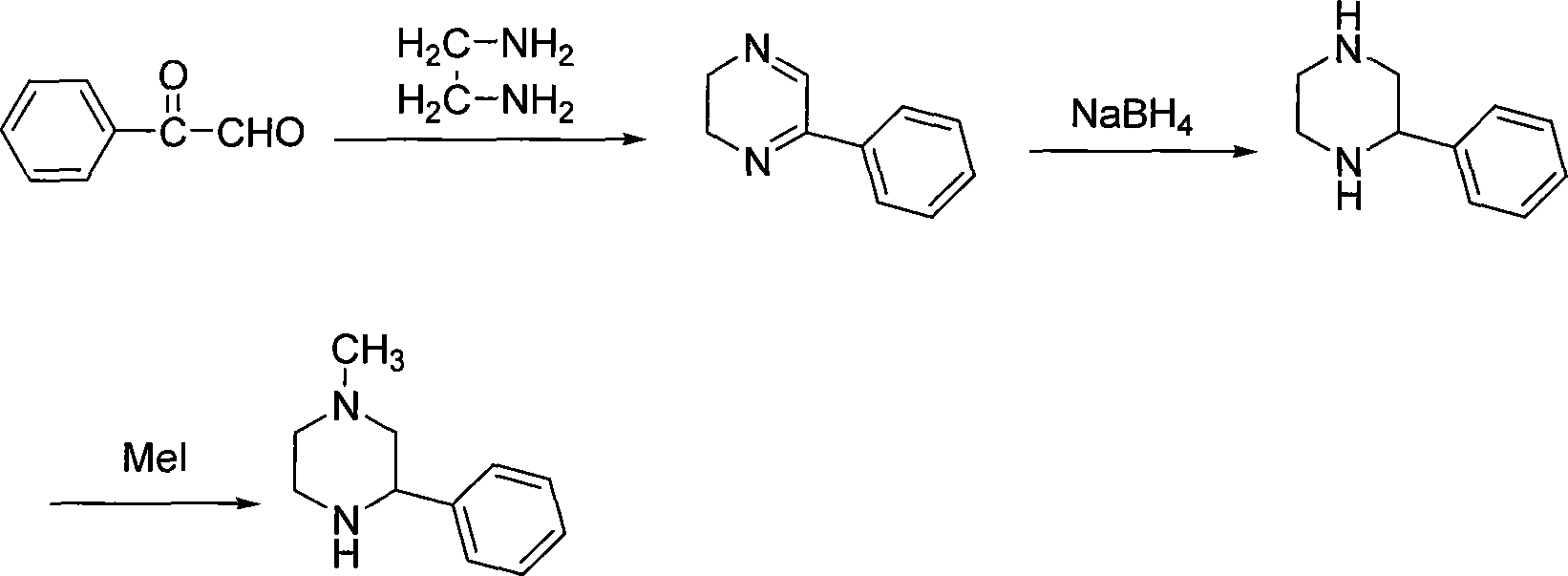
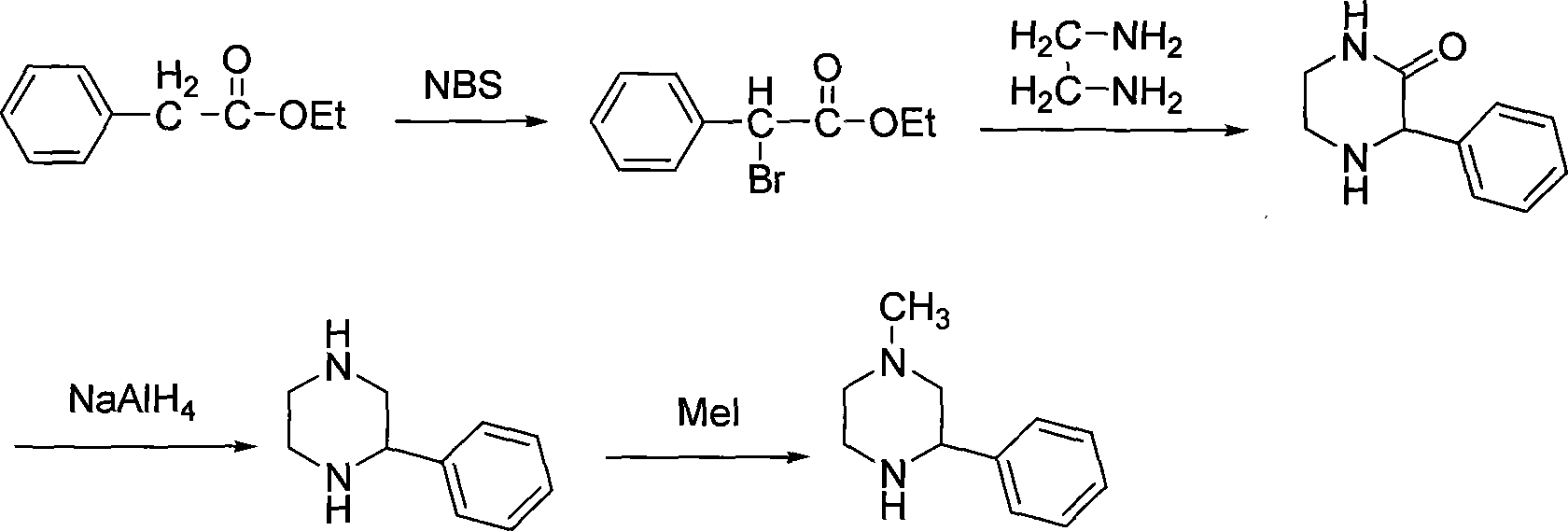
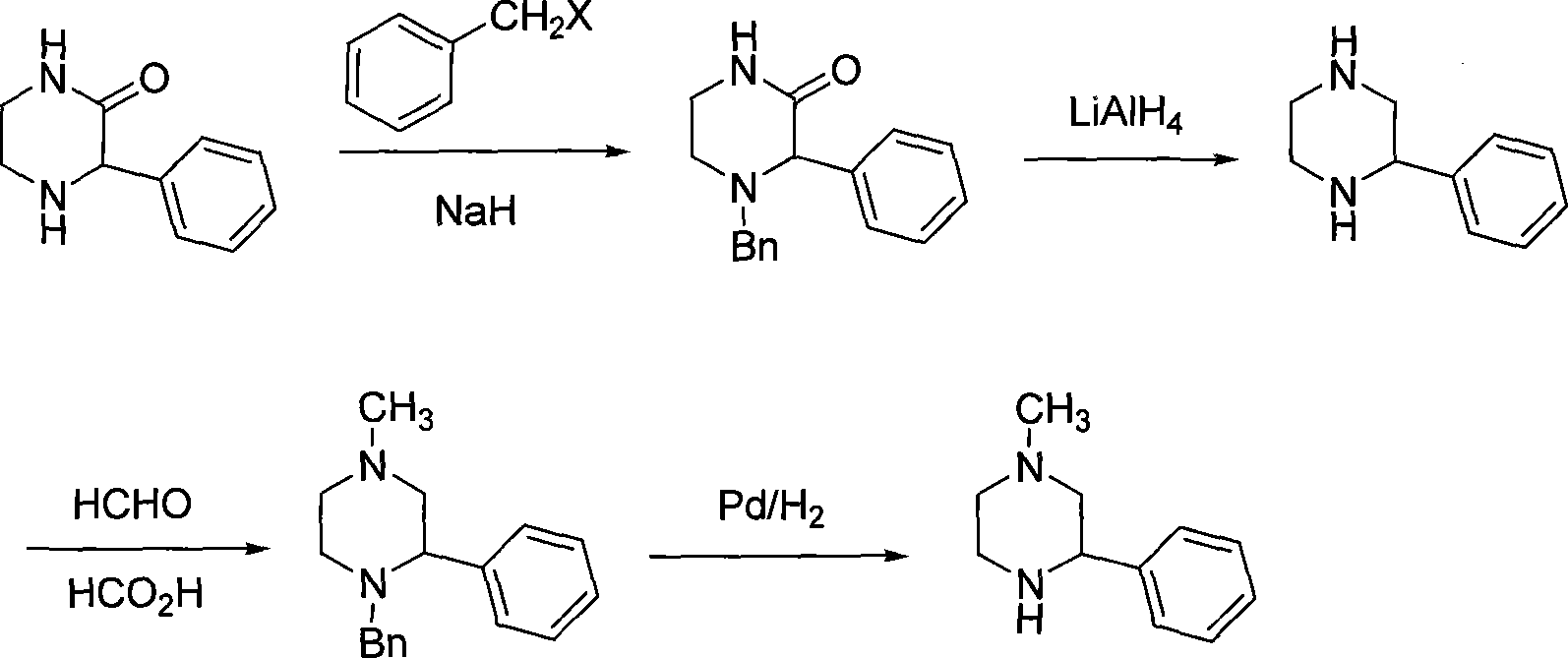
Preparation of medicament intermediate 1-methyl-3-phenyl piperazine
A technology for phenylpiperazine and intermediates, which is applied in the field of preparation of pharmaceutical intermediates 1-methyl-3-phenylpiperazine, can solve problems such as low yield, and achieve high reaction yield, good quality, and easy operation convenient effect
Active Publication Date: 2010-08-25
上海津力药业股份有限公司
View PDF11 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The design idea of this method can be used for reference, but the flammable and explosive NaH and ether are used, and the yield is too low, the two-step yield is only 58.1%
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention relates to a method for preparing a medicine intermediate, namely 1-methyl-3-phenyl-piperazine. Epoxy phenylethane and N-methyl ethanolamine react for ring opening in a methylbenzene solution and further react with thionyl chloride to obtain N-(2- chloroethyl)-N-methyl-2-chloro-2-phenylethylamine hydrochloride, the hydrochloride reacts with toluene sulfonamide and sodium hydroxide in a DMF solution to obtain 1-methyl-4-p-toluenesufonyl-3-phenylpiperazine, the 1-methyl-4-p-toluenesufonyl-3-phenylpiperazine reacts with concentrated hydrochloric acid to remove sulfonyl, and a product is obtained through neutralization extraction and recrystallization. Compared with the prior art, the method for preparing the 1-methyl-3-phenyl-piperazine has the advantages of low cost of selected raw materials, cost conservation, few synthesis steps, convenient and safe operation, simple post-treatment, high reaction yield, high product purity, good quality, and suitability for industrialized application.
Description
technical field The invention relates to a pharmaceutical intermediate, in particular to a preparation method of a pharmaceutical intermediate 1-methyl-3-phenylpiperazine. Background technique 1-methyl-3-phenylpiperazine (hereinafter referred to as MPP) is a key intermediate for the preparation of mirtazapine. Mirtazapine (mirtazapine) is an antidepressant drug (U.S.4062848). It is a selective serotonin reuptake inhibitor (SSRI). Clinical use in many countries of the world. So far, the methods for preparing MPP mainly contain the following: 1. Maeda, C.JP2001122863 reported that phenylglyoxal was used as a raw material, aminated and reduced to obtain 2-phenylpiperazine, and then methylated to obtain MPP. The synthetic route of this method is as follows: The raw materials used in this route are expensive, and the reaction yield is low, especially in the last step of methylation, there are several possible by-products, the yield is low, and it is not easy to purify. 2....
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07D241/04
Inventor 潘朝阳胡惟孝武克孟跃杨忠愚戴龙华
Owner 上海津力药业股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com