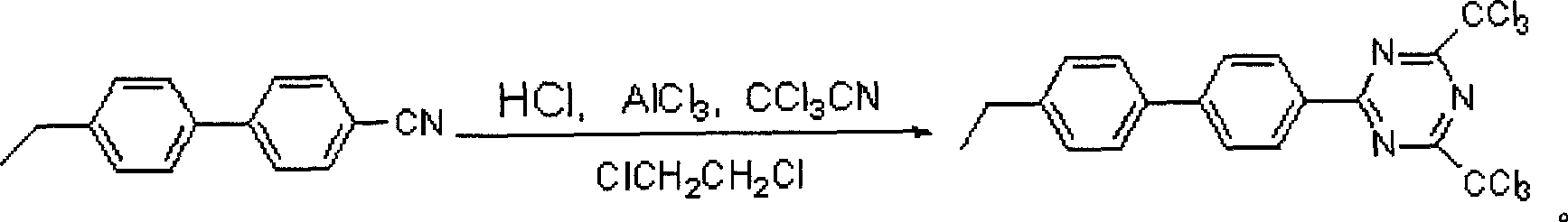Method for synthesizing 4,6-bis(trichloromethyl)-2-p-acetyl biphenyl-1,3,5-triazine
A bis-trichloromethyl and acetyl biphenyl technology, applied in the direction of organic chemistry, can solve the problems of harsh reaction conditions, environmental pollution, cumbersome post-treatment, etc., and achieve the effect of mild reaction conditions, good yield and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] A method for synthesizing 4,6-bistrichloromethyl-2-p-acetylbiphenyl-1,3,5-triazine, carried out according to the following steps:
[0021] a 1 .4,6-bistrichloromethyl-2-p-ethylbiphenyl-1,3,5-triazine preparation: add 0.02mol of p-ethylbiphenonitrile and 0.05mol of trichloroacetonitrile into the four-necked flask, Add 0.004mol of anhydrous aluminum trichloride, add 20.00mL of 1,2-dichloroethane as a solvent, stir, cool down the ice bath to 0°C, pass in dry HCl gas, remove the ice bath after 5h, and stir at room temperature for 20h. Excess trichloroacetonitrile and 1,2-dichloroethane were distilled off under reduced pressure, and the residue B was subjected to column chromatography to obtain 4.31 g of white solid A, namely 0.009 mol of 4,6-bistrichloromethyl-2-para Ethylbiphenyl-1,3,5-triazine, yield 44.00%.
[0022] 1 H-NMR (CDCl 3 , 500MHz):
[0023] 1.299 (t, 3H, CH 3 ), 2.726 (q, 2H, CH 2 ), 7.352(d, 2H, 4 CH), 7.635(d, 2H, 3 CH), 7.812(d, 2H, 2 CH), 8.751(d...
Embodiment 2
[0028] A method for synthesizing 4,6-bistrichloromethyl-2-p-acetylbiphenyl-1,3,5-triazine, carried out according to the following steps:
[0029] a 2 .4,6-bistrichloromethyl-2-p-ethylbiphenyl-1,3,5-triazine preparation:
[0030] Add 0.02mol of p-ethylbiphenylnitrile as raw material, 0.05mol of trichloroacetonitrile, 0.004mol of anhydrous aluminum trichloride, and 20.00mL of solvent 1,2-dichloroethane into a four-neck flask, stir, and drop the ice bath to 0 ℃, continue to feed dry HCl gas, remove the ice bath after 5h, and stir at room temperature for 24h. Excess trichloroacetonitrile and 1,2-dichloroethane were distilled off under reduced pressure, and the residue was subjected to column chromatography to obtain 4.31 g of white solid A, namely 0.009 mol of 4,6-bistrichloromethyl-2-p-ethane Biphenyl-1,3,5-triazine.
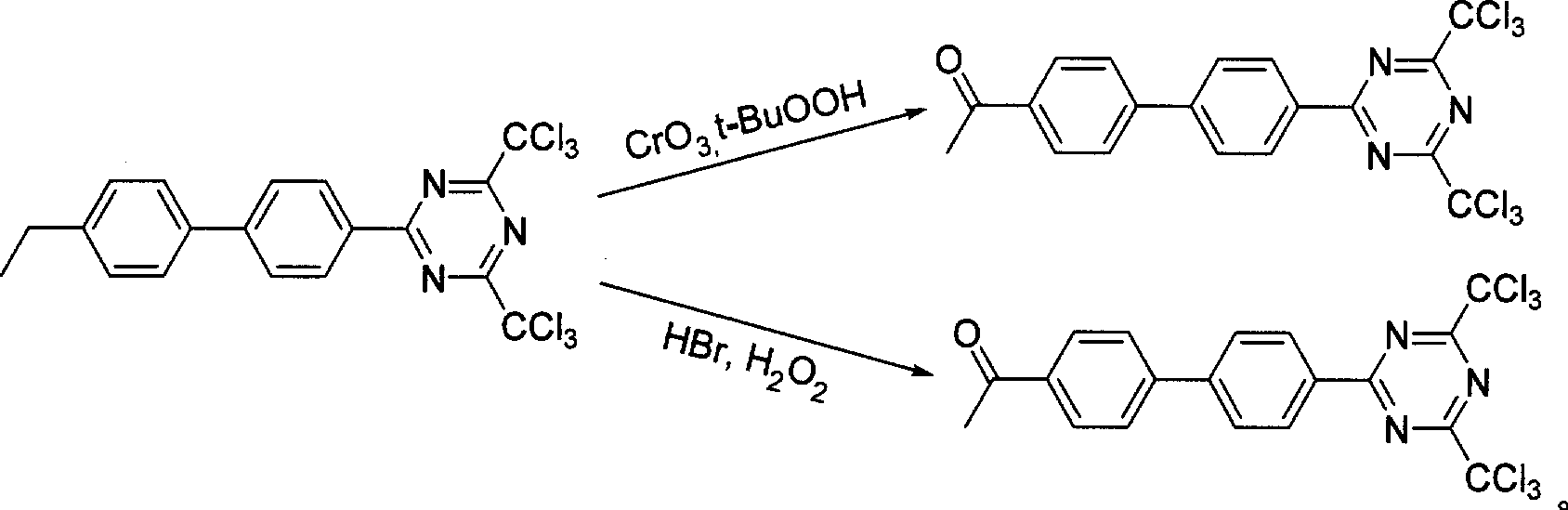
[0031] b 2 .4,6-bistrichloromethyl-2-p-acetylbiphenyl-1,3,5-triazine preparation: the obtained 4.31g white solid A and solvent dichloromethane 22.00mL, 30% H ...
Embodiment 3
[0033] A method for synthesizing 4,6-bistrichloromethyl-2-p-acetylbiphenyl-1,3,5-triazine, carried out according to the following steps:
[0034] a 3.4,6-bistrichloromethyl-2-p-ethylbiphenyl-1,3,5-triazine preparation: add 0.39mol of p-ethylbiphenonitrile and 0.97mol of trichloroacetonitrile into the four-necked flask, Add 0.078 mol of anhydrous aluminum trichloride, add 386.00 mL of 1,2-dichloroethane as a solvent, stir, cool down the ice bath to 0°C, pass in dry HCl gas, remove the ice bath after 20 h, and stir at room temperature for 18 h. Excessive trichloroacetonitrile and 1,2-dichloroethane were distilled off under reduced pressure, and the residue B was recrystallized from methanol and dichloromethane to obtain a white solid A with a weight of g 1 =49.40g; the crystallization mother liquor was placed at 0°C for 24h, and the weight was obtained in g 2 =38.10 g white solid A, yield 45.93%.
[0035] This step receives the total weight g=g of white solid A that is 4,6-bi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com