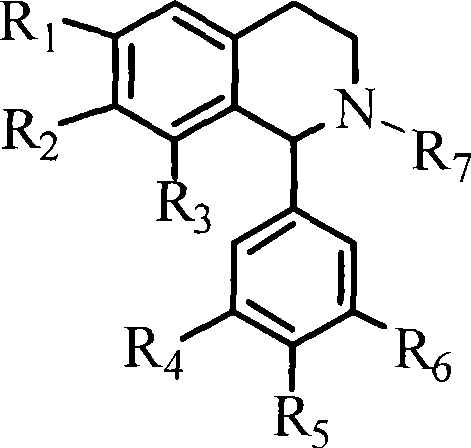
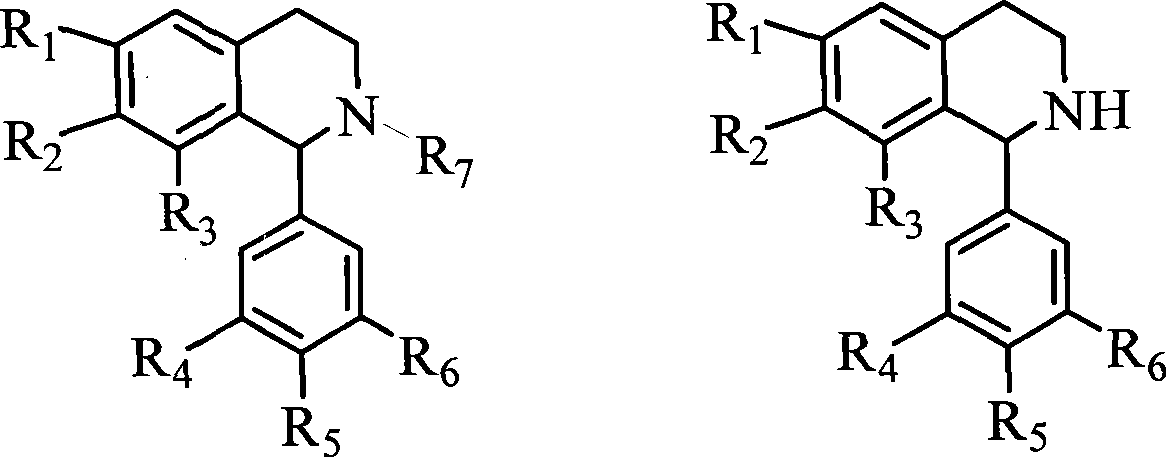
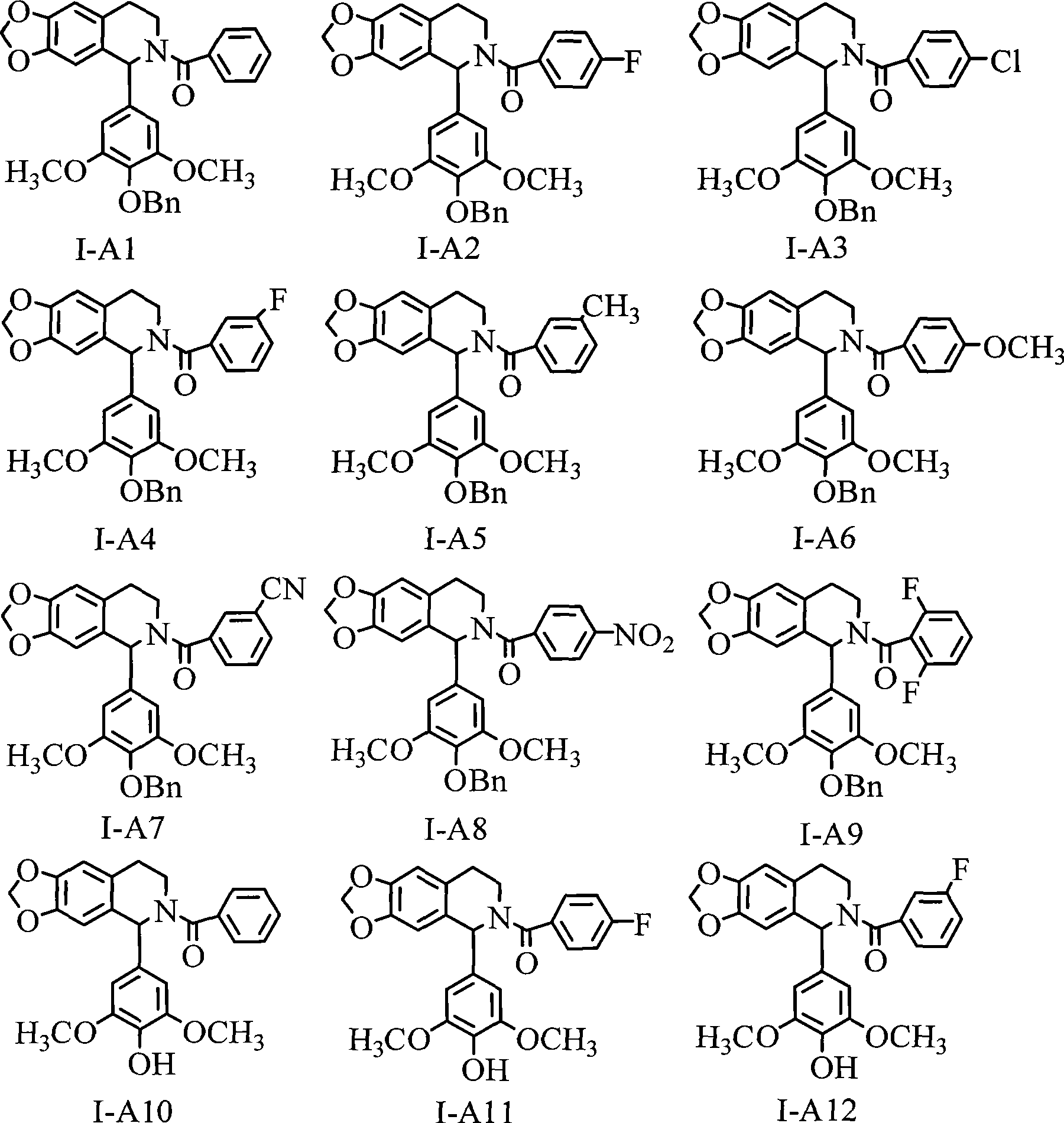
1-(3',4',5'- trisubstituted phenyl)- tetrahydroisoquinoline compound and its use
A technology of tetrahydroisoquinoline and compounds, applied in the field of medicinal chemistry and pharmacology, can solve problems such as limiting the universal applicability of drugs, low selectivity, and malignant killing of normal cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0128] Preparation example 1: Preparation of compound IV-1 [N-(3-benzyloxy)phenethyl-(3',5'-dimethoxy-4'-benzyloxy)benzamide]:
[0129]
[0130] Dissolve 4.4g (16.0mmol) of 3,5-methoxy-4-benzyloxy-benzoic acid in 200ml of dry dichloromethane, add 20ml (230mmol) of oxalyl chloride dropwise under ice-cooling conditions, and complete the reaction at room temperature After 2 hours, excess oxalyl chloride was distilled off under reduced pressure. The residue was redissolved in 50ml of dry dichloromethane, and added dropwise to a solution of 4.5g (19.8mmol) of 3-benzyloxyphenethylamine and 0.5ml of triethylamine in 200ml of dichloromethane under ice-cooling conditions After stirring at room temperature overnight, add 50ml of 10% sodium hydroxide solution, stir for 30 minutes, separate the organic phase, and wash with saturated sodium carbonate (50ml×2), water (50ml×2) and saturated saline (50ml×2) respectively. )washing. After drying over anhydrous magnesium sulfate and evapor...
preparation example 2—13
[0134] Preparation example 2-13: Preparation 2-13 compounds shown in the following table 1 are prepared according to the method with the above preparation example 1:
[0135]
[0136] Table I
[0137]
[0138] Listed below is the physicochemical data of each compound in Table 1:
[0139] IV-2: jelly, Rf (ethyl acetate / petroleum ether 2:1): 0.12; 1 H NMR (400Mz, CD 3 OD): δ 2.82 (t, 2H, J=7.7, 7.0Hz, CH 2 -Ar), 3.55(t, 2H, J=7.3, 7.4Hz, CH 2 -NH), 3.87(s, 6H, OCH 3 ×2), 6.62-6.72(m, 3H, H-2, H-6), 7.08(d, 1H, J=7.7Hz, H-5), 7.12(s, 2H, H-2′, H- 6').
[0140] IV-3: white solid, Rf (ethyl acetate / petroleum ether 1:1): 0.73; 1 H NMR (400MHz, CDCl 3 ): δ 2.89 (t, 2H, J=6.9, 6.5Hz, CH 2 -Ar), 3.66(q, 2H, J=6.4, 6.9, 6.2Hz, CH 2 -NH), 3.82(s, 6H, OCH 3 ×2), 5.04(s, 2H, OCH 2 Ph), 5.05(s, 2H, OCH 2 Ph), 6.20 (brs, 1H, NH), 6.89 (s, 2H, H-2′, H-6′), 6.94 (dd, 2H, J=6.5, 2.0Hz, H-3, H-5) , 7.17 (dd, 2H, J=6.6, 2.0Hz, H-3, H-5), 7.27-7.47 (m, 10H, OCH 2 Ph-H×2).
...
preparation example 14
[0151] Preparation Example 14: Preparation of compound III-A1 [6-benzyloxy-1-(3',5'-dimethoxy-4'-benzyloxyphenyl)-3,4-dihydroisoquinoline]:
[0152]
[0153] Suspend 638 mg (1.28 mmol) of compound (IV-1) (see Preparation 1) in 50 ml of toluene, add 0.25 ml (2.6 mmol) of phosphorus oxychloride, reflux for 4 hours, cool to room temperature, and evaporate the toluene under reduced pressure , dissolve the residue in 30ml ethyl acetate, adjust the pH to 8-9 with concentrated ammonia water under ice-bath cooling, separate the water layer, extract with ethyl acetate (10ml×2), combine the organic phases, and successively water (10ml×2) 2), washed with saturated brine (10ml×2), dried over anhydrous sodium sulfate. After filtration and concentration, the crude product was separated and purified by column chromatography (eluent: ethyl acetate / petroleum ether 1:2) to obtain 370 mg of solid with a yield of 60.3%.
[0154] III-A1 compound:
[0155] Rf (ethyl acetate / petroleum ether 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com