Method for stabilizing color and luster, moisture and fluohydric acid content of non-aqueous electrolyte
A non-aqueous electrolyte, hydrofluoric acid technology, applied in non-aqueous electrolyte batteries, electrolyte battery manufacturing, circuits, etc., can solve the problems of darkening of color, discoloration of non-aqueous electrolyte, affecting the use effect, etc., so that the color does not occur. effect of change
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
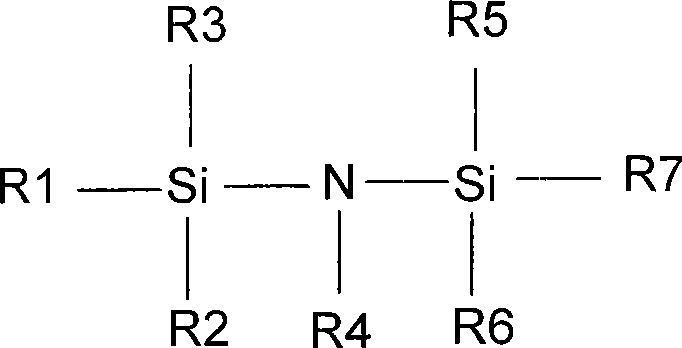
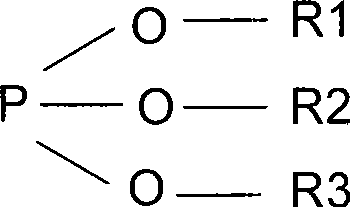
[0019] Prepare non-aqueous electrolyte 1M LiPF in an argon-filled glove box (moisture 6 , EC: DMC: EMC=1: 1: 1 (mass ratio), four parts in total, each part of 1L, taking the original sample without additives as the standard, adding hexamethyldisilazane and ethylene oxide respectively in the remaining three samples Tributyl phosphate 0.02%, 0.4%, 1%, after sealing, conduct a comparative test at 50°C to detect the content and color of water and hydrofluoric acid, the results are shown in the table below.
[0020]
[0021]
[0022] It can be seen from the above table that after adding an appropriate amount of hexamethyldisilazane and tributyl phosphite to the non-aqueous electrolyte, the content of water and hydrofluoric acid remains stable and the color is stable compared with the original state without addition.
Embodiment 2
[0024] Prepare non-aqueous electrolyte 1M LiPF in an argon-filled glove box (moisture 6 , EC:DMC:EMC=1:1:1 (mass ratio), a total of three parts, each 1L, with no additives as the standard, the remaining two samples were added 0.5% of hexamethyldisilamine alkane and tributyl phosphite, 0.5% hexaethyldisilazane and triphenyl phosphite, after sealing, conduct a comparative test at 50°C to detect the content and color of moisture and hydrofluoric acid, and the results See the table below.
[0025]
[0026] It can be seen from the above table that after adding an appropriate amount of silicon amine compounds and phosphite compounds to the non-aqueous electrolyte, compared with the original without addition, the content of water and hydrofluoric acid remains stable, and the color is stable.
Embodiment 3
[0028] Prepare non-aqueous electrolyte 1M LiPF in an argon-filled glove box (moisture 6 , EC:DMC:EMC=1:1:1 (mass ratio), a total of six parts, each 1L, with no additives as the standard (three parts), the remaining three samples were added 0.3% Hexa Disilazane and tributyl phosphite, after sealing, conduct a comparative test at 50°C to 70°C to detect the content and color of water and hydrofluoric acid, the results are shown in the table below.
[0029]
[0030] It can be seen from the above table that after adding an appropriate amount of silicon amine compounds and phosphite compounds to the non-aqueous electrolyte, compared with the original solution without adding it, the moisture and The content of hydrofluoric acid remains stable and the color does not change.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com