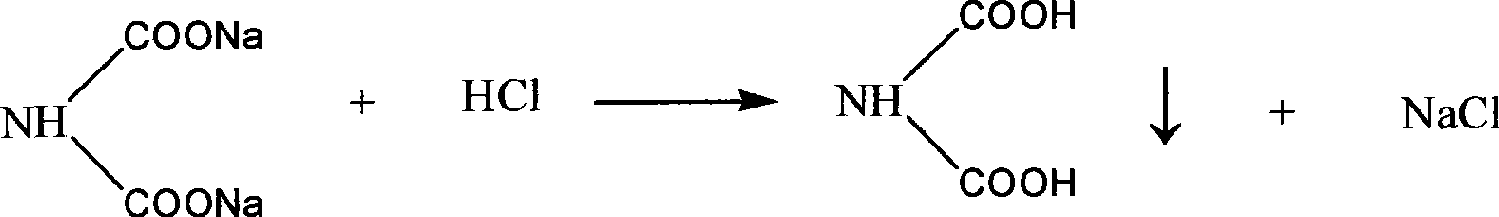Method for preparing iminodiacetic acid from iminodiacetic acid disodium salt
A technology of disodium iminodiacetic acid and sodium iminodiacetic acid, which is applied in the field of preparation of iminodiacetic acid, can solve the problems of low operating efficiency, large equipment investment, cumbersome process, etc., and achieve saving investment and energy consumption, and production cycle Short, easy-to-use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
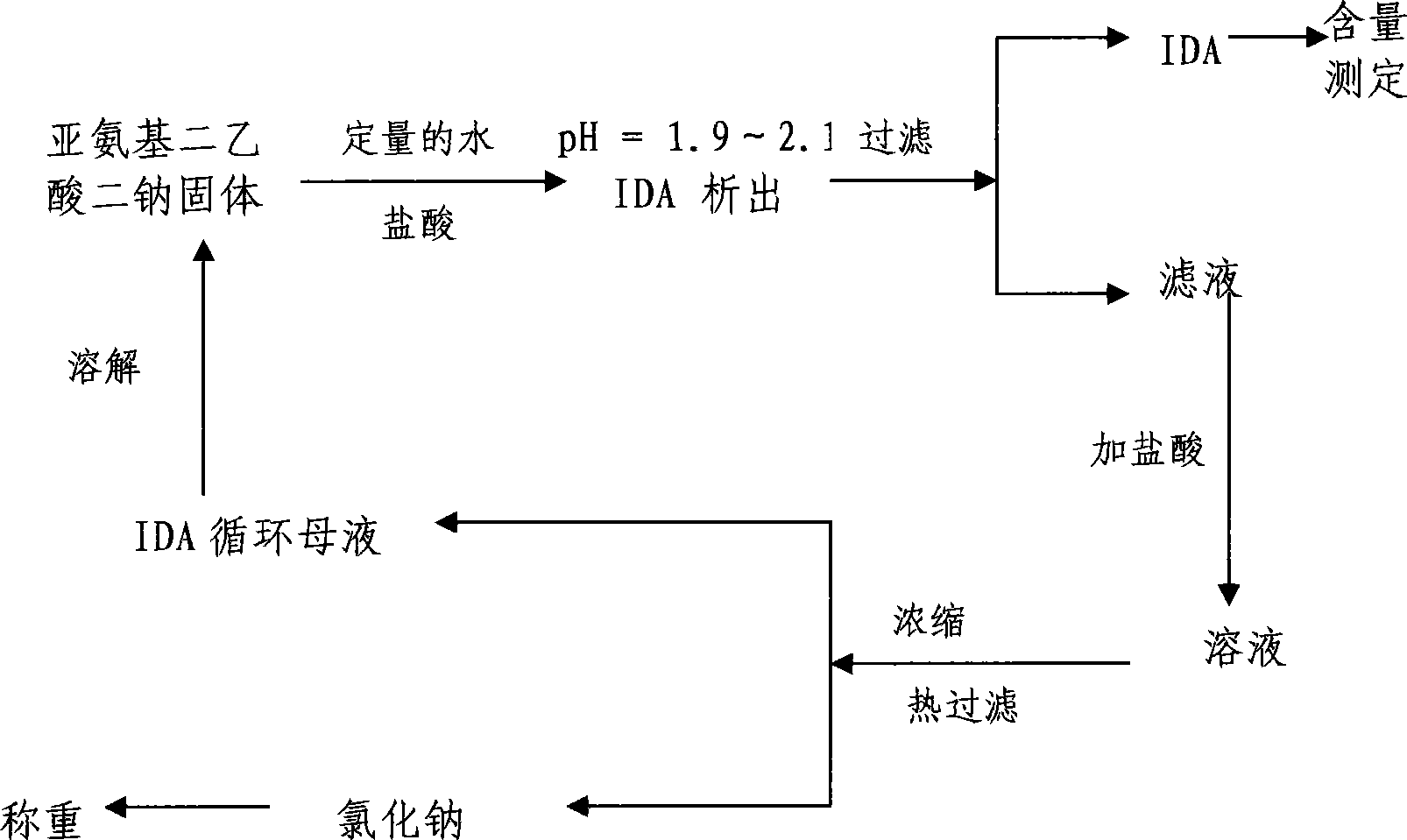
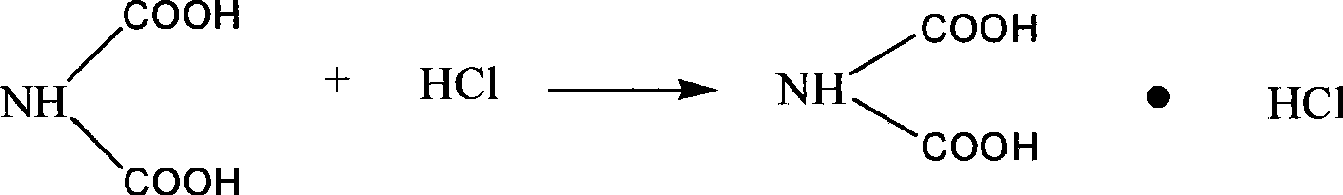
[0029] Weigh 200g of disodium iminodiacetic acid solid, add 150ml of tap water, stir and dissolve at 35-40°C, then slowly drop about 70ml into the filtrate with a stirring speed of 100r / min and a flow rate of 10ml / min. The mass concentration is 35-37 % hydrochloric acid, adjust pH = 1.98, iminodiacetic acid crystallizes out, let it stand for 10 minutes, filter to obtain IDA, dry at 100-105°C, and weigh to obtain a qualified product. Filtrate pH = 2.3, add 50ml of hydrochloric acid with a mass concentration of 35-37% to the filtrate, adjust pH = 1.35, concentrate in vacuo until a large amount of salt precipitates, filter with suction, dry and weigh the obtained salt and detect its content, and the filtrate is circulated to dissolve The second batch of iminodiacetic acid disodium solids, and thus repeated circulation 15 times. In the experiment, the total yield of IDA was 97.6%, the content was 98.7%, and the salt removal rate was 99.2%.
Embodiment 2
[0031] Weigh 200g of disodium iminodiacetic acid solid, add 180ml of tap water, stir and dissolve at 35-40°C, then slowly drop about 70ml into the filtrate with a stirring speed of 200r / min and a flow rate of 10ml / min. The mass concentration is 35-37 % hydrochloric acid, adjust the pH=1.95, iminodiacetic acid crystals precipitate out, stand still for 20 minutes, filter to obtain IDA, dry at 100-105°C, and weigh to obtain a qualified product, the filtrate pH=2.4. Add 50ml of hydrochloric acid with a mass concentration of 35-37% to the filtrate, adjust pH=1.32, concentrate in vacuo until a large amount of salt precipitates, filter with suction, dry and weigh the obtained salt, and detect its content, and the filtrate circulates to dissolve the second batch of sub Aminodiacetic acid disodium solid, and thus repeated cycle 25 times. In the experiment, the total yield of IDA was 98.1%, the content was 98.5%, and the salt removal rate was 98.2%.
Embodiment 3
[0033]Weigh 200g of disodium iminodiacetic acid solid, add 150ml of tap water, stir and dissolve at 35-40°C, then slowly drop about 70ml into the filtrate with a stirring speed of 200r / min and a flow rate of 10ml / min. 37% hydrochloric acid, adjust pH=1.98, iminodiacetic acid crystallizes out, stand still for 15 minutes, filter to obtain IDA, dry at 100-105°C, weigh to obtain qualified product, filtrate pH=2.5. Add 60ml of hydrochloric acid with a mass concentration of 35-37% to the filtrate, adjust pH=1.30, concentrate in vacuo until a large amount of salt is precipitated, filter with suction, dry and weigh the obtained salt, and detect its content, and circulate the filtrate to dissolve the second batch of sub Aminodiacetic acid disodium solid, and thus repeated 15 times. In the experiment, the total yield of IDA was 97.9%, the content was 98.3%, and the salt removal rate was 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com