Method for extracting noble metal iridium from mixed oxide containing ruthenium, iridium, titanium, tin, zirconium and palladium
A mixed oxide and precious metal technology, applied in the direction of improving process efficiency, can solve the problems of low output and high price, and achieve the effect of saving energy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
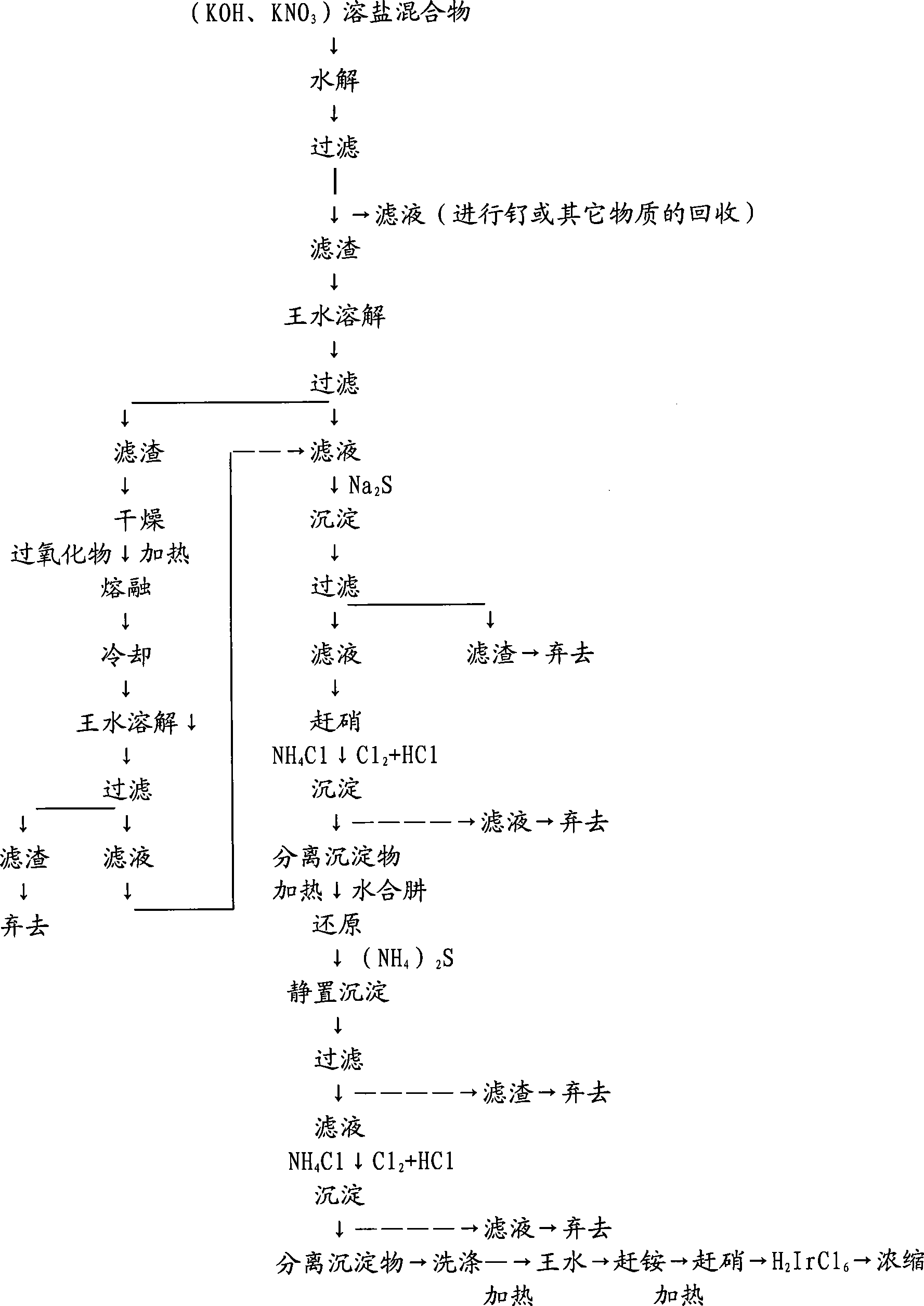
[0035] Take an electrode 1m containing mixed oxide coatings such as ruthenium, iridium, titanium, zirconium, and tin 2 , which contained iridium 1.1g after testing.
[0036] 1 square meter of the above iridium-containing coating was placed in KOH and KNO in a weight ratio of 1:3 3 Dissolve in mixed molten salt, the dissolution temperature is 400°C, then cool the molten salt, soak in water, filter and separate; heat the filtered filter residue to 90°C with aqua regia to melt, so that IrO 2 Generate dark red soluble H 2 IrCl 6 .
[0037] Then after dilution and filtration, the filtrate is retained, the filter residue is dried, collected in a magnetic crucible and melted with hydrogen peroxide, the melting temperature is 650°C, and melted for 1 hour. After cooling, it is immersed in water and then heated with aqua regia at 60-70°C to dissolve and filter. The filtrate was combined with the previously retained filtrate, and the filter residue was discarded. The combined filtra...
Embodiment 2
[0043] Take the mixed oxide coated electrode containing noble metal iridium 1m 2 , It contains 0.98g of iridium after detection.
[0044] 1 square meter of the above iridium-containing coating was placed in KOH and KNO in a weight ratio of 1:2 3 Dissolve in mixed molten salt, the dissolution temperature is 350°C, then cool the molten salt, soak in water, filter and separate; heat the filtered filter residue with aqua regia to 95°C to melt, so that IrO 2 Generate dark red soluble H 2 IrCl 6 .
[0045] Then after dilution and filtration, the filtrate is retained, the filter residue is dried, collected in a magnetic crucible and melted with hydrogen peroxide, the melting temperature is 655°C, and melted for 0.5 hours. After cooling, it is immersed in water and then heated with aqua regia at 60-70°C to dissolve and filter. The filtrate was combined with the previously retained filtrate, and the filter residue was discarded. The combined filtrates now contained chloroiridic ac...
Embodiment 3
[0051] Take the mixed oxide coated electrode containing noble metal iridium 1m 2 , It contains iridium 1.05g after detection.
[0052] Dissolve 1 square meter of the above-mentioned iridium-containing coating in KOH and KNO3 molten salt at a temperature of 430°C, then cool the molten salt, soak it in water, filter and separate it; heat the filtered filter residue to 85°C with aqua regia °C melts, making IrO 2 Generate dark red soluble H 2 IrCl 6 .
[0053] Then after dilution and filtration, the filtrate is retained, the filter residue is dried, collected in a magnetic crucible and melted with hydrogen peroxide, the melting temperature is 645°C, and melted for 1.5 hours. After cooling, it is immersed in water and then heated with aqua regia at 60-70°C to dissolve and filter. The filtrate was combined with the previously retained filtrate, and the filter residue was discarded. The combined filtrates now contained chloroiridic acid and other mixtures, and Na 2 S precipitat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com