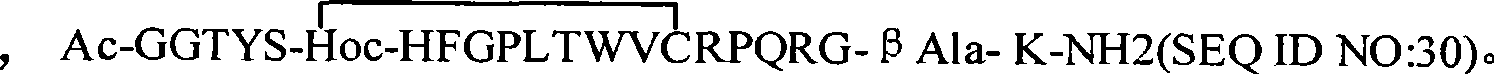Erythrocyte-stimulating factor mimic peptide derivative, medical salts thereof, preparation method and use thereof
A technology for erythropoietin and derivatives, applied in the field of erythropoietin mimetic peptide derivatives and pharmaceutically acceptable salts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1: Synthesis of Erythropoietin Mimetic Peptide Derivative Monomer Peptide
[0071] The synthesis of monomeric peptides of erythropoietin-mimetic peptide derivatives adopts the method of solid-phase peptide synthesis. This type of peptide synthesis method has been reported in many literatures. See stewart, J.M., and Young, J.D., solid phase peptide synthesis 2dedition, novabiochem peptide synthesis notes. The erythropoietin mimetic peptide derivative monomer peptide provided by the present invention adopts the method of manual synthesis, the resin is rink amind resin, and the α amino group of the amino acid derivative used is formed by Fmoc (fluorenyl carbonyl) Protection, cysteine side chain sulfhydryl group, glutamine side chain amino group, histidine side chain imidazolyl group are protected by Trt (trityl), arginine side chain guanidine group is protected by Pbf(2,2,4, 6,7-pentamethyldihydrobenzofuran—5-sulfonyl) protection, tryptophan side chain indole gro...
Embodiment 2
[0072] Example 2: Preparation of functional small molecule (LG-1)
[0073]
[0074] Step 1: Preparation of LG-1-A
[0075]Dissolve diethyl iminodiacetate (10.0g, 52.8mmol), Boc-β-alanine (10.0g, 52.8mmol) in 100mL of dichloromethane, add DIC (8.0mL, 52.8mmol), Stir at room temperature overnight, filter, and the filtrate is successively washed with 100ml saturated NaHCO 3 , 50ml of 0.5N HCl solution, washed with 100ml of saturated brine, separated the organic layer, and the organic layer was washed with anhydrous MgSO 4 dry. Filter and concentrate to obtain LG-1-A as a colorless oily liquid: 17g.
[0076] Step 2: Preparation of LG-1-B
[0077] Dissolve 17 g of LG-1-A in 100 mL of MeOH: THF = 1:1 mixture, add 25 mL of water and 5 g of NaOH (125 mmol). After stirring at room temperature for 2 h, the pH value was adjusted to 1 with 6N HCl solution. Extracted 4 times with ethyl acetate. The organic layer was washed with brine, dried over anhydrous magnesium sulfate, and c...
Embodiment 3
[0082] Example Three: Preparation of Functional Small Molecules (LG-2)
[0083]
[0084] Dissolve 4g LG-1-B (13mmol) in 100mL N,N-dimethylformamide, add hydroxysuccinimide (3.1g, 21mmol), DIC (4mL, 26mmol) and DMAP (4-dimethyl Aminopyridine) (12mg, 0.08mmol). Stir overnight and concentrate under reduced pressure. The residue was dissolved in 80 ml of ethyl acetate, and the insoluble matter was filtered off. The organic phase was washed once with 40 ml of saturated sodium bicarbonate solution, 40 ml of saturated saline, 40 ml of 0.5N HCl solution, and 40 ml of saturated brine, and the organic layer was separated and dried over anhydrous magnesium sulfate. After filtration, the filtrate was concentrated under reduced pressure to obtain 4.4 g of white solid LG-2 (the yield was about 68%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com