Method for controlling quality of anti-inflammation antibacterial medicament
A quality control method and preparation technology, applied in antibacterial drugs, drug combinations, pharmaceutical formulations, etc., can solve the problems of poor control of product quality, poor specificity, complex components, etc., and achieve easy operation, good separation, Methods with high precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] A quality control method for Yankening preparations, comprising the following steps:
[0070] (1) Identification:
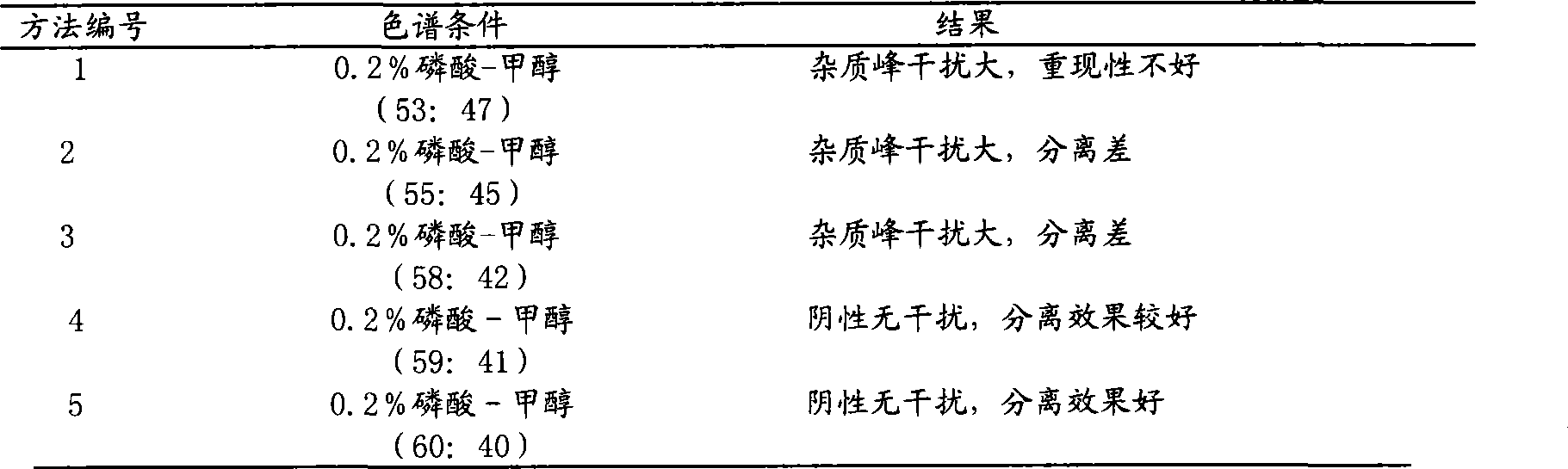
[0071] a. Identification of rhubarb: Weigh 0.1g of this product powder, add 20ml of methanol, soak for 1 hour, filter, take 5ml of filtrate, evaporate to dryness, add 10ml of water to the residue to dissolve, add 1ml of hydrochloric acid, heat and reflux for 30 minutes, immediately Cool, shake and extract twice with diethyl ether, 20ml each time, combine the diethyl ether solution, evaporate to dryness, add 1ml of chloroform to the residue to dissolve, and use it as the test solution; take another 0.1g of rhubarb reference medicine, and make the same method as the reference medicine solution; test according to thin-layer chromatography (Appendix VI B), draw 4 μl of the above-mentioned test product and 3 μl of the reference medicinal material, respectively spot on the same silica gel G thin-layer plate, and use petroleum ether (30~60°C)-ethyl formate- The ...
Embodiment 2-10
[0081] Same as Example 1
[0082] The results are shown in Table 8
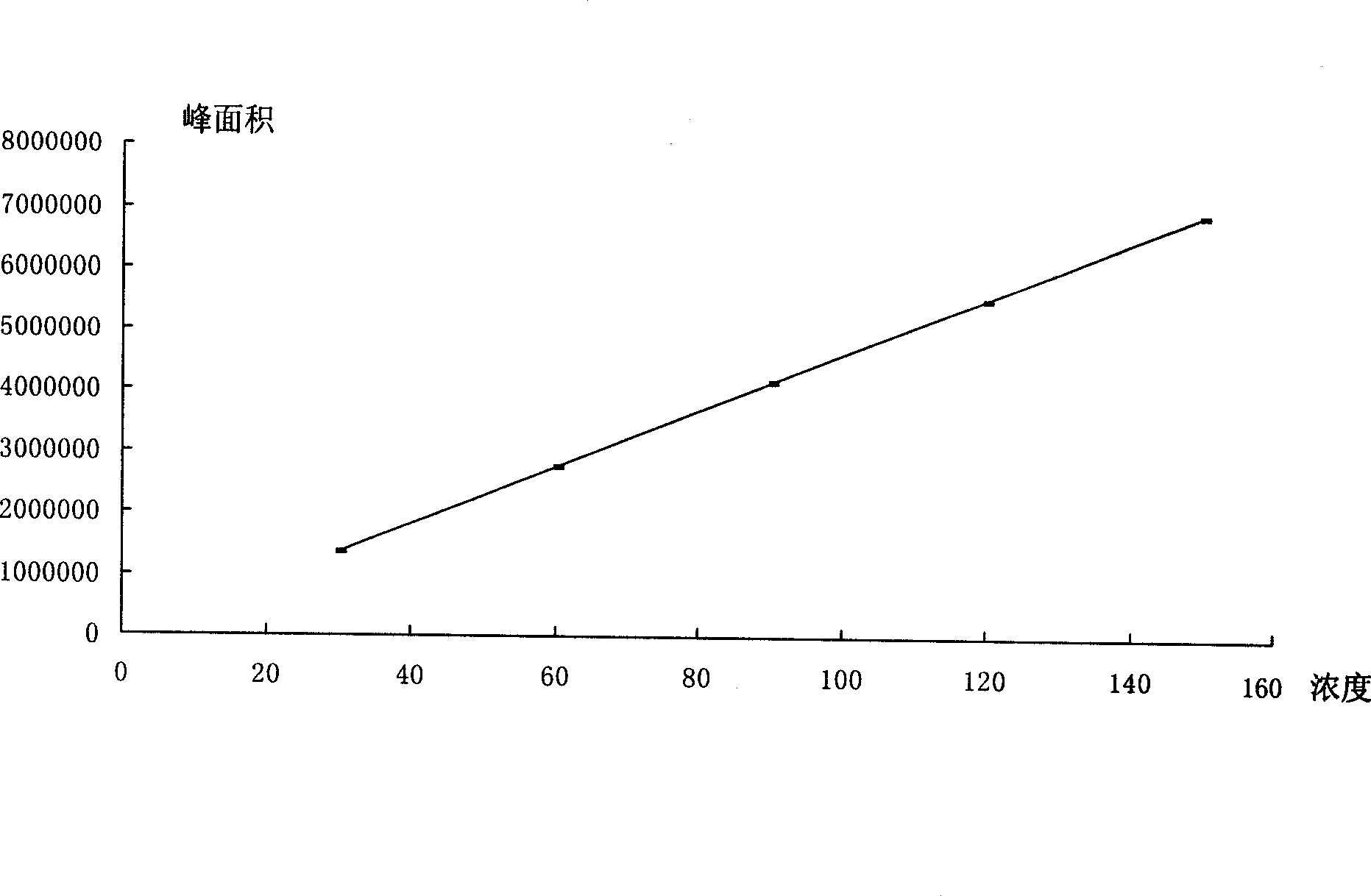
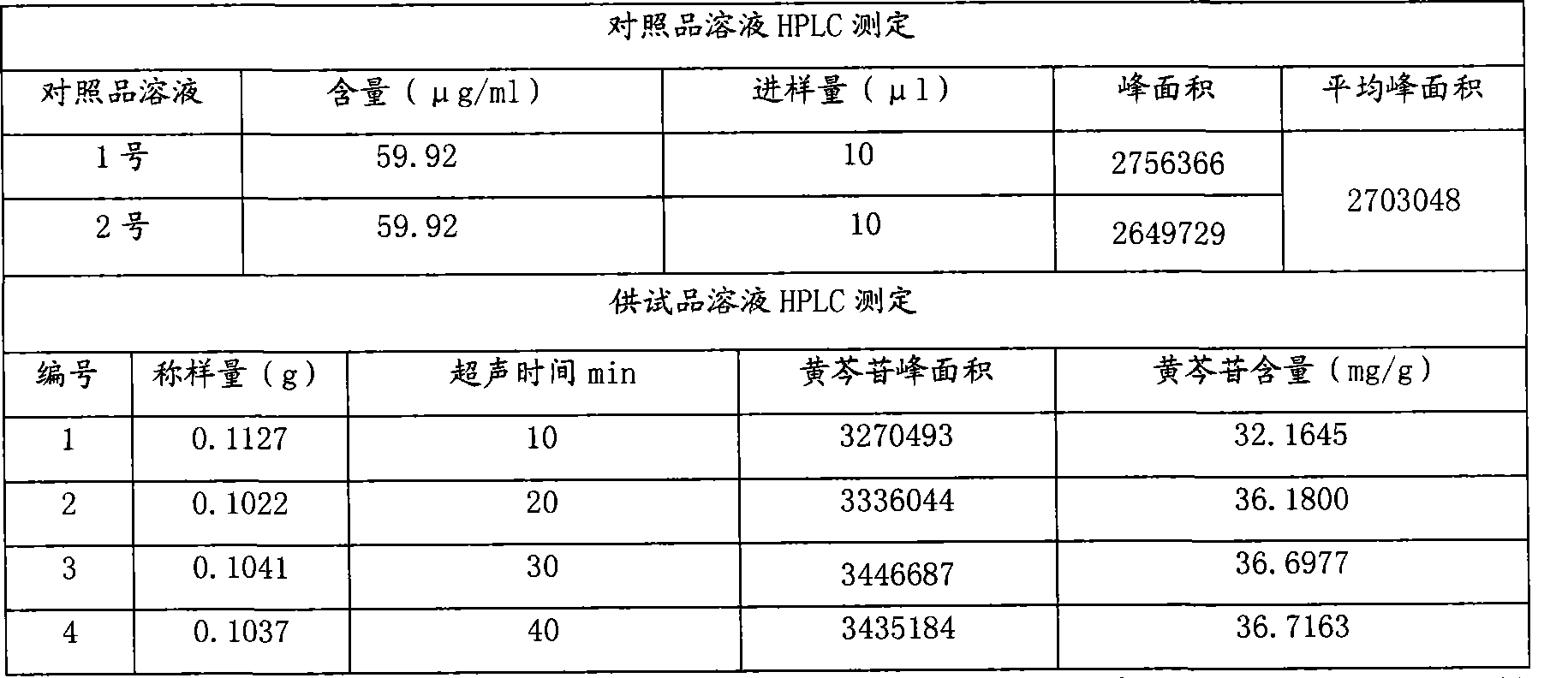
[0083] Table 8 Statistical Table of Sample Baicalin Content Investigation
[0084]
[0085] The content limit of baicalin in Yankening Tablets (mg / g) = the dosage of Scutellaria baicalensis (g) x the content limit of baicalin in Scutellaria baicalensis (mg / g) x average yield (%) ÷ the amount of finished product (g) = 310.3g ×90mg / g×35.27%÷340g=28.97mg / g, namely: 9.85mg / tablet.
[0086] It can be seen that each tablet of this product contains Scutellaria baicalensis and baicalin (C 21 h 18 o 11 ) shall not be less than 9.85mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com