A lithium ion battery electrolyte and a lithium ion battery containing the electrolyte
A lithium-ion battery and electrolyte technology, applied in secondary batteries, circuits, electrical components, etc., can solve hidden dangers, high and low temperature safety problems, etc., and achieve the effects of preventing expansion, widening the liquid temperature range, and reducing the amount of gas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] This example is used to illustrate the electrolyte solution provided by the present invention.
[0042] (1) Preparation of ionic liquid
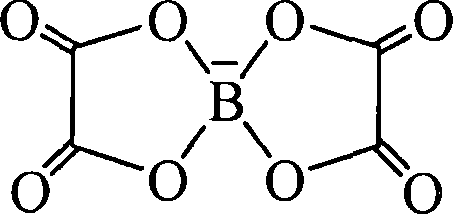
[0043] Under the protection of nitrogen, with 30 ml of acetonitrile as solvent, 20.4 g of N-methylpiperidine and 28.2 g of bromobutane were refluxed at 70°C for 4 hours, filtered, and vacuum-dried at 50°C for 5 hours to obtain N- Methyl-N-butylpiperidinium bromide. Take 30.0 grams of the obtained N-methyl-N-butylpiperidine bromide and 24.6 grams of lithium bisoxalate borate at room temperature for 3 hours, after liquid separation and evaporation of the solvent, vacuum dry at 90 ° C for 10 hours to obtain N - Bisoxalate borate of methyl-N-butylpiperidine (PP14BOB).
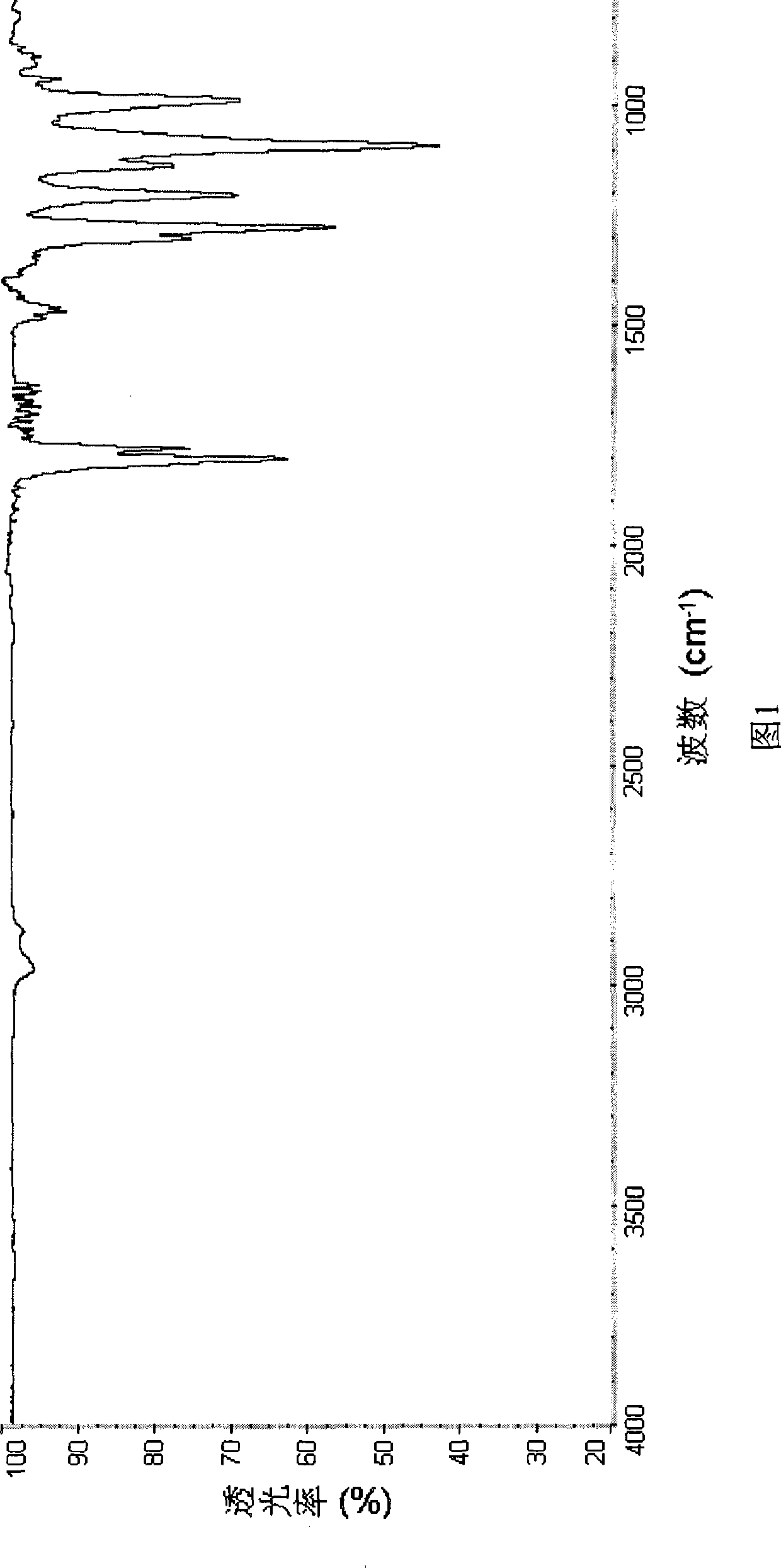
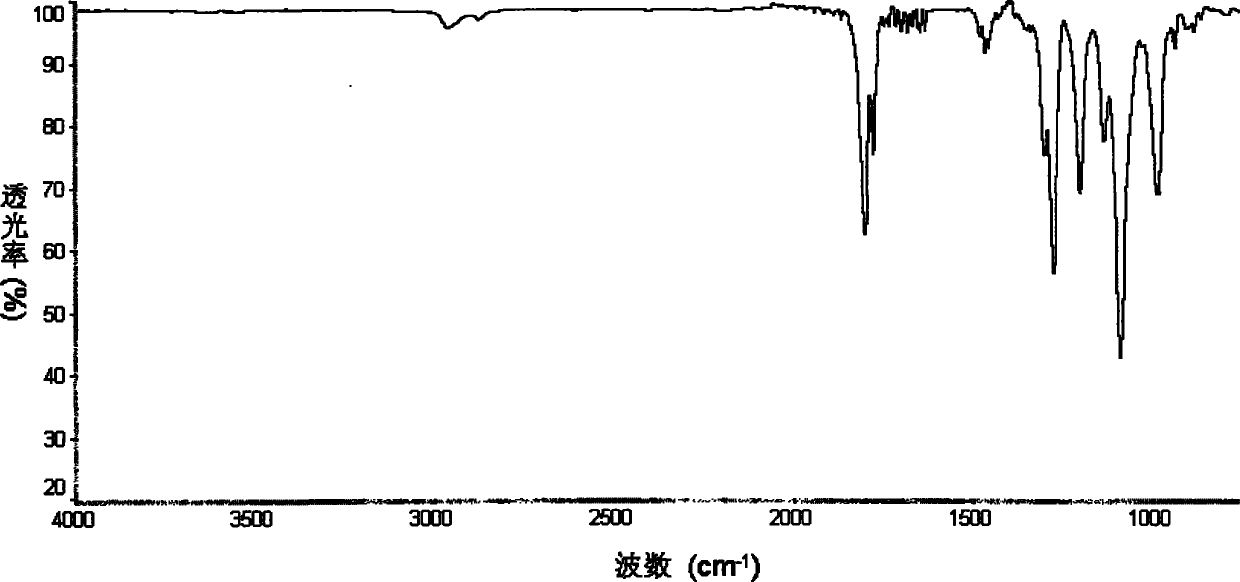
[0044] The obtained ionic liquid was separated and purified by silica gel column chromatography, and then stored in a glove box. figure 1 is the FTIR image of the obtained PP14BOB, where the wavenumber is 1275cm -1 The peak is the stretching vibration peak of the B-O s...
Embodiment 2
[0048] This example is used to illustrate the electrolyte solution provided by the present invention.
[0049] (1) Preparation of ionic liquid
[0050] Prepare the bisoxalate borate (Py13BOB) of N-methyl-N-propylpyrrole according to the same method as in Example 1 step (1), except that 17.5 grams of N-methylpyrrole replaces 20.4 gram of N-methylpiperidine, 25.3 grams of bromopropane instead of 28.2 grams of bromobutane.
[0051] (2) Preparation of electrolyte
[0052] At room temperature, in a glove box, Py13BOB, propylene carbonate, and ethyl methyl carbonate were mixed in a weight ratio of 30:35:35, and then LiPF was added to it 6 The electrolyte is formulated into an electrolyte solution with a concentration of 0.6 mol / liter. The prepared electrolyte sample is denoted as A2.
Embodiment 3
[0054] This example is used to illustrate the electrolyte solution provided by the present invention.
[0055] (1) Preparation of ionic liquid
[0056] Prepare trimethylpropylammonium dioxalate borate (N1113BOB) in the same manner as in Example 1 step (1), except that 12.1 grams of trimethylamine replace 20.4 grams of N-methylpiperine Pyridine, 25.3 grams of bromopropane instead of 28.2 grams of bromobutane.
[0057] (2) Preparation of electrolyte
[0058] At room temperature, in a glove box, N1113BOB, propylene carbonate, and ethyl methyl carbonate were mixed in a weight ratio of 50:25:25, and then LiPF was added to it 6 The electrolyte is formulated into an electrolyte solution with a concentration of 1.5 mol / liter. The prepared electrolyte sample is denoted as A3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com