Tetracaine hydrochloride lipidosome gel and preparation method thereof
A technology of tetracaine hydrochloride fat and tetracaine hydrochloride, which is applied in the direction of medical formula, medical preparations with no active ingredients, medical preparations containing active ingredients, etc., can solve the problem of inability to reduce the toxic and side effects of tetracaine hydrochloride, Short time and other issues, to achieve the effect of transparent and good-looking appearance, convenient drug use, and soft skin touch
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Weigh 260 mg of tetracaine hydrochloride, 433 mg of carbomer, 1213 mg of azone, 8663 mg of propylene glycol, 2599 mg of glycerin, 520 mg of triethanolamine, 1733 mg of soybean lecithin, and 347 mg of cholesterol. Or weigh 520 mg of tetracaine hydrochloride, 693 mg of carbomer, 1559 mg of azone, 9529 mg of propylene glycol, 3119 mg of glycerin, 866 mg of triethanolamine, 2079 mg of soybean lecithin, and 520 mg of cholesterol.
[0030] The above-mentioned soybean lecithin and cholesterol were dissolved in chloroform and placed in a 250mL pear-shaped bottle. On a rotary evaporator, the water bath was at 40°C for 20 minutes to form a film and the chloroform was removed. Hydrate with ammonium sulfate solution for 30 minutes, and pass through the membrane with an extruder at 0.8um, 0.45um, 0.22um, and 0.1um respectively. Put this blank liposome into a dialysis bag, dialyze in 500mL normal saline, 37 ℃ circulating water bath for 17h, take out the liposome after dialysis, put i...
Embodiment 2
[0032] Release test of tetracaine hydrochloride liposome and tetracaine hydrochloride aqueous solution
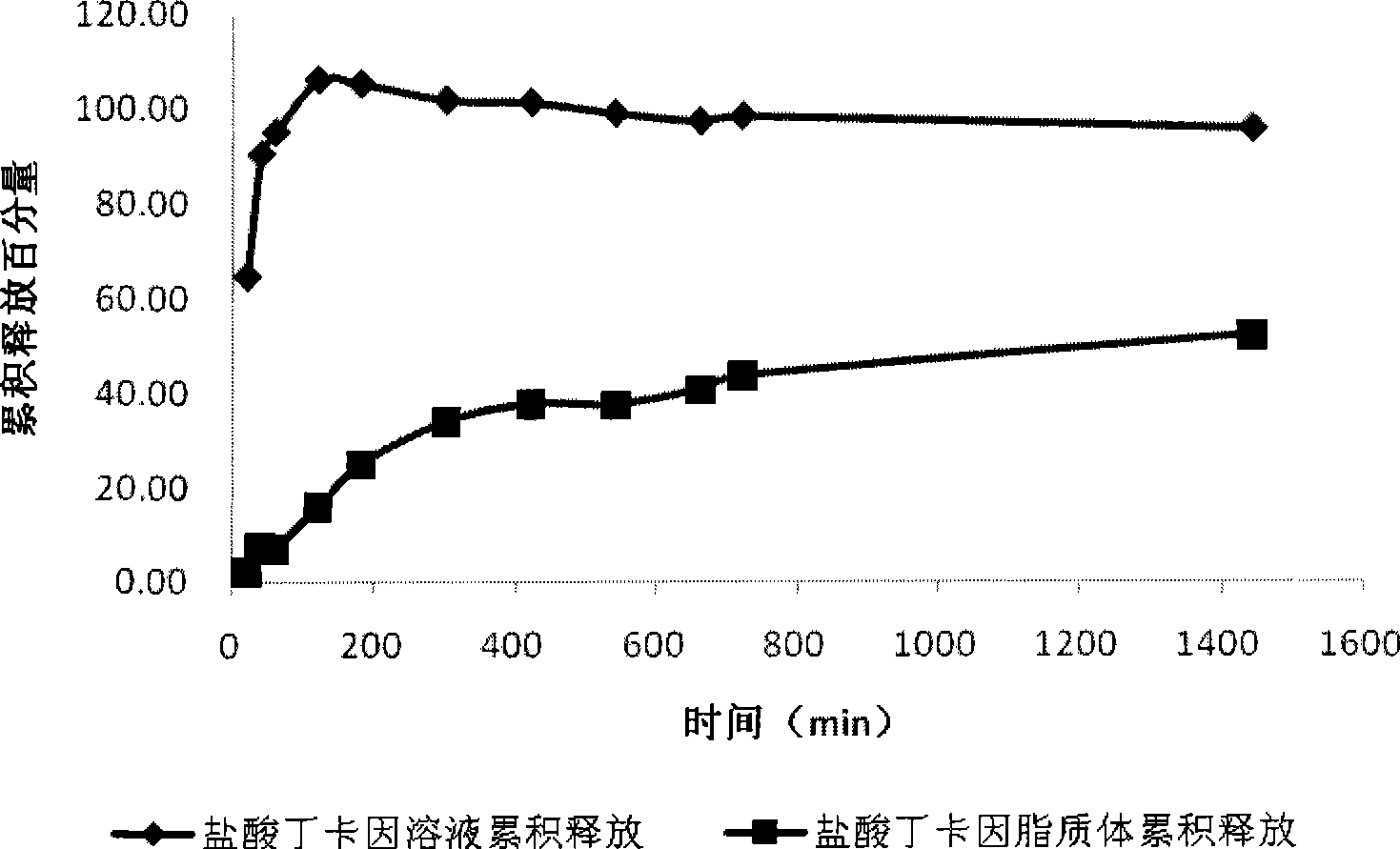
[0033] Put tetracaine hydrochloride liposome 5mL in the dialysis bag (tetracaine hydrochloride concentration is about 5.56mg / mL), the corresponding concentration of the prepared drug solution is 5.56mg / mL5mL and puts in the dialysis bag, respectively simultaneously in the stripping bag that 250mL distilled water is housed Timing release in the cup, speed 100r.min -1 , temperature 37°C. Take samples at 20min, 40min, 1h, 2h, 3h, 5h, 7h, 9h, 11h, 12h, 24h respectively, measure its concentration with HPLC, draw the release curve, calculate the cumulative release amount and draw, tetracaine hydrochloride liposome and The 24h cumulative release of tetracaine hydrochloride aqueous solution see figure 1 .
[0034] Depend on figure 1 It can be seen that tetracaine hydrochloride solution releases faster, and in the time of 2h, the cumulative release amount of tetracaine hydrochlo...
Embodiment 3
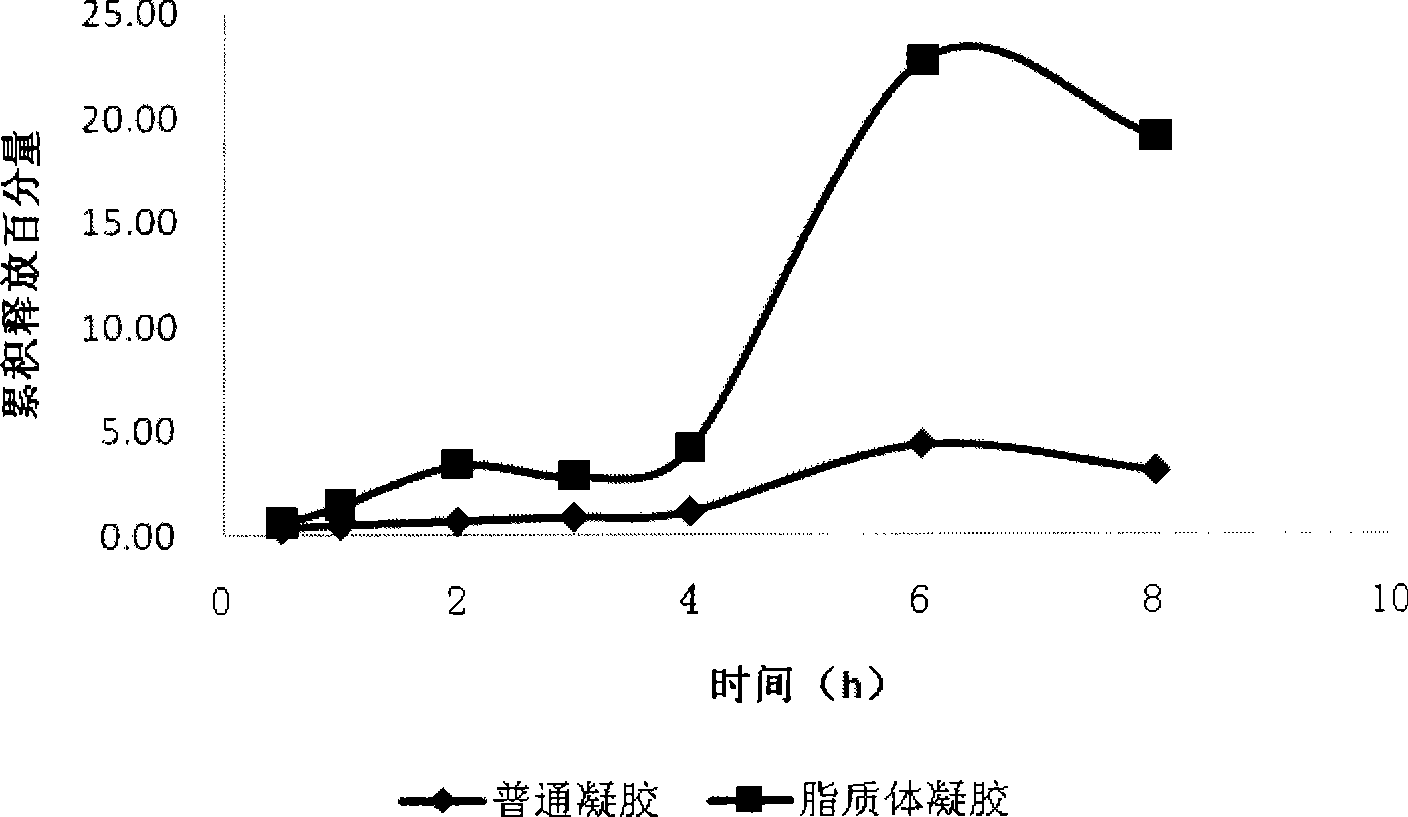
[0036] Comparison of drug solution and liposome cumulative release:
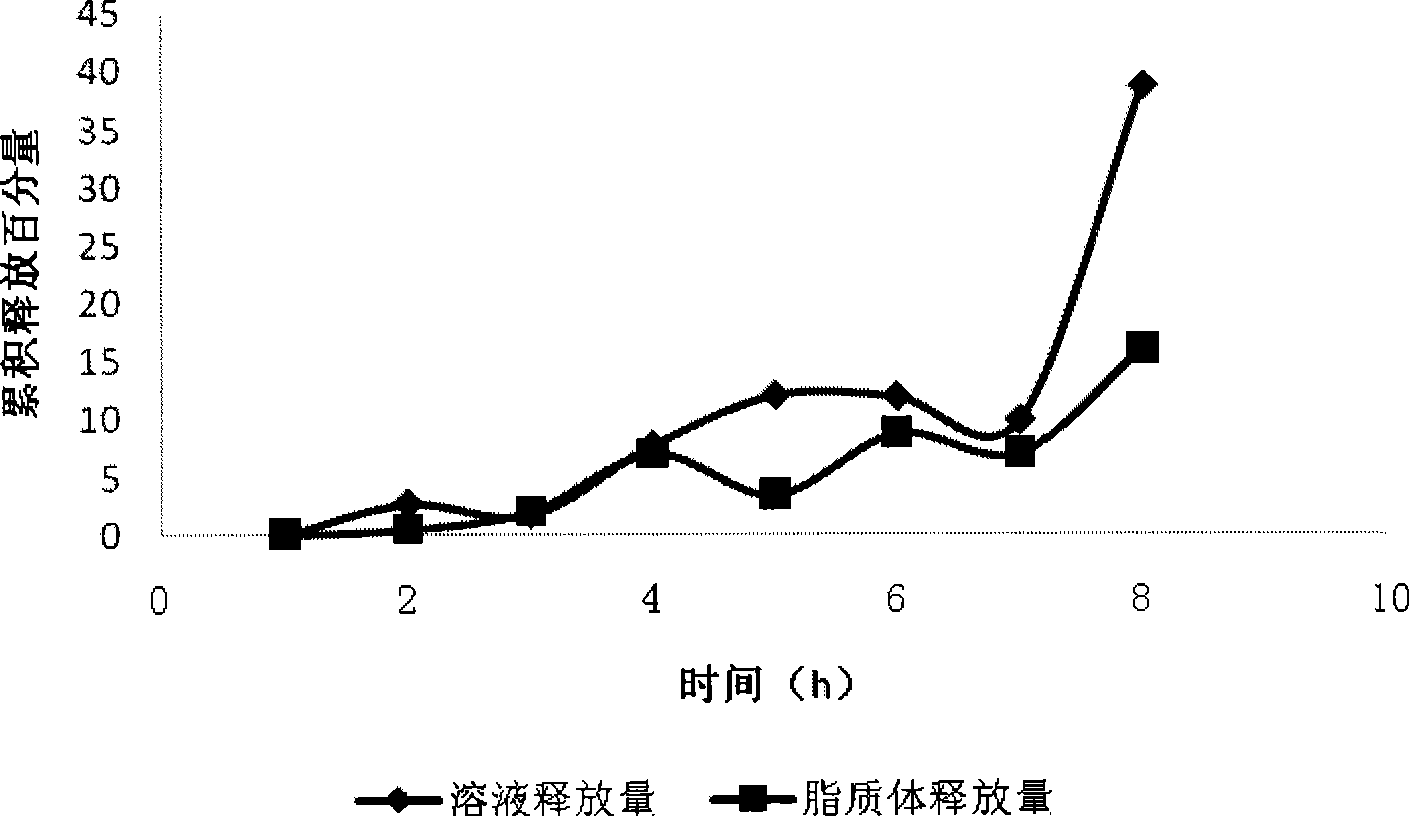
[0037] Franz diffusion cell was used to compare the release of drug solution and liposome. Load 0.5mL samples respectively, take samples at 0.5h, 1h, 2h, 3h, 4h, 6h, and 8h, and calculate the concentration and cumulative release, see figure 2 .
[0038] Depend on figure 2 It can be seen that the release of the drug solution is faster than that of the liposome, and the release amount is larger, which is also the reason why the drug solution fails quickly and cannot last too long. Liposome release is slow and stable.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com