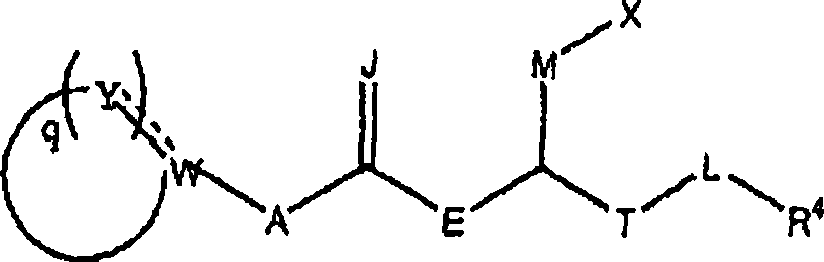
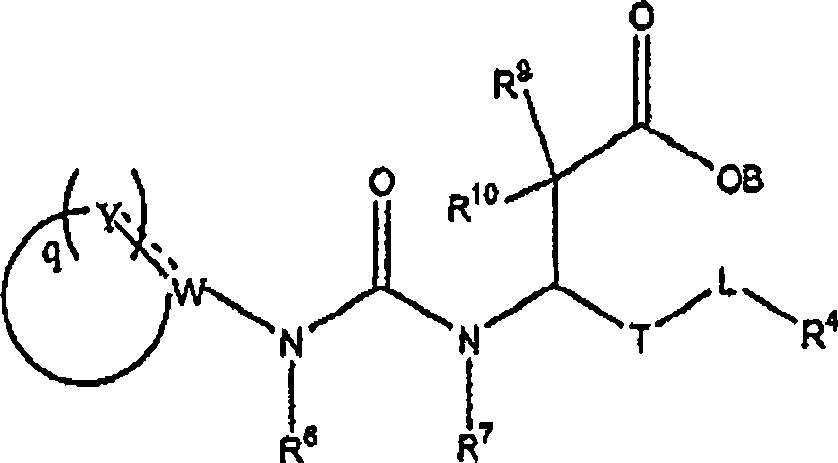
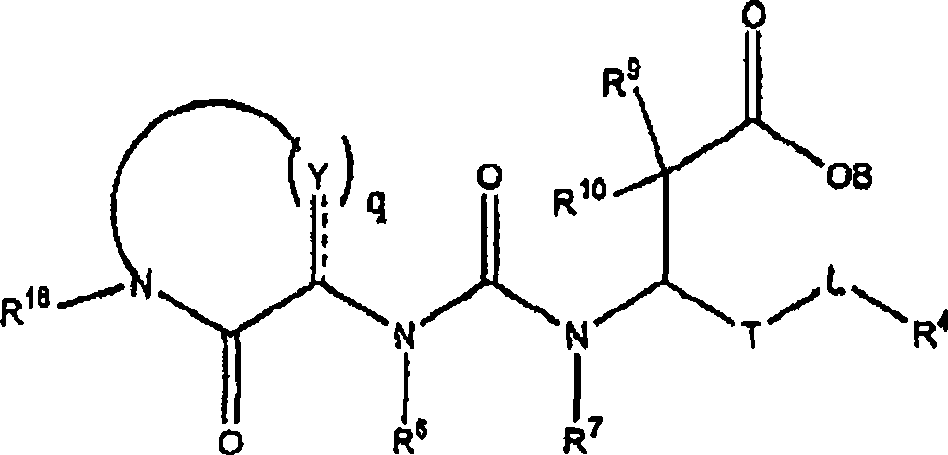
Carboxylic acid derivatives inhibiting the binding of integrins to receptors thereof
A technology of derivatives and compounds, applied in the field of compounds that inhibit this binding, can solve the problems of leukocyte influx, uncontrollable leukocyte migration, tissue damage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0584] (3S)-3-{[({1-[(2-chlorophenyl)methyl]-4-ethyl-2-oxo-1,2-dihydro-3-pyridyl}amino)carbonyl Synthesis of ]amino}-3-(4-methylphenyl)propionic acid (10)
[0585] step 1: Compound 1 (20.8 g, 135 mmol) was dissolved in methanol (270 mL), and palladium on carbon (10% palladium dry weight basis, Degussa E101 NE / W type, ~50% water content, 5.75 g, 2.7 mmol Pd) was added . The atmosphere was replaced with hydrogen (switching between vacuum and balloon hydrogen 5 times), the mixture was stirred overnight, then filtered. The filtrate was concentrated in vacuo, the residue was taken up with a 1:1 hexane:ethyl acetate mixture, water and saturated NaHCO 3 , saturated NaHCO 3 Wash with a 4:1 mixture of brine. The organic layer was treated with MgSO 4 After drying and filtering, the filtrate was concentrated under reduced pressure to obtain compound 2 (12.43 g, 74%) as a white solid. This material was used without purification.
[0586] Step 2: Compound 2 (2.64 g, 21.3 mmol) w...
Embodiment 2
[0594] (3S)-3-{[({6-Methyl-2-oxo-1-(benzyl)-4-[(benzyl)oxy]-1,2-dihydro-3-pyridine Synthesis of yl}amino)carbonyl]amino}-3-(4-methylphenyl)propionic acid (15)
[0595] step 1: To compound 11 (1.0g, 5.9mmol) and K 2 CO 3 Benzyl bromide (2.31 g, 13.5 mmol) was added to a suspension of (2.40 g, 17.6 mmol) in acetone (50 mL). After reflux overnight, the reaction was cooled and the mixture was partitioned between ethyl acetate and saturated NaHCO 3 between. The organic layer was washed with dilute HCl and brine, washed over MgSO 4 After drying and filtering, the filtrate was concentrated to obtain compound 12 (1.60 g, 80%).
[0596] Step 2: Compound 12 (0.30g, 0.86mmol), zinc powder (0.30g, 4.6mmol) and saturated NH 4 Aqueous Cl (0.30 mL) was mixed in MeOH (18 mL). The mixture was stirred at room temperature for 1 hour, then additional zinc powder (0.30 g, 4.6 mmol) was added. The resulting heterogeneous mixture was refluxed overnight. After filtering the hot mixture a...
Embodiment 3
[0600] (3S)-3-{[({4-amino-1-[(2-chlorophenyl)methyl]-6-methyl-2-oxo-1,2-dihydro-3-pyridyl Synthesis of}amino)carbonyl]amino}-3-(4-methylphenyl)propionic acid (22)
[0601] step 1: To a solution of compound 11 (10.00 g, 58.8 mmol) in anhydrous DMF (120 mL) was added NaH (60% dispersion in mineral oil, 5.40 g, 135 mmol) at 0°C. The mixture was stirred at 0°C for 15 minutes, then 2-chlorobenzyl chloride (12.3 g, 76.4 mmol) was added. After stirring overnight at 55°C, the mixture was poured into ice water and washed with Et 2 O washed twice. The aqueous layer was acidified and the resulting precipitate was filtered to give compound 16 (14.7 g, 85%).
[0602] Step 2: Under a dry nitrogen atmosphere at room temperature, the flask containing compound 16 (8.00 g, 28.6 mmol) was sealed with a rubber septum and a balloon, and POCl was added via a syringe. 3 (30.0ml, 322mmol). The nitrogen delivery line was removed and the reaction mixture was stirred overnight at 70°C, then pou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com