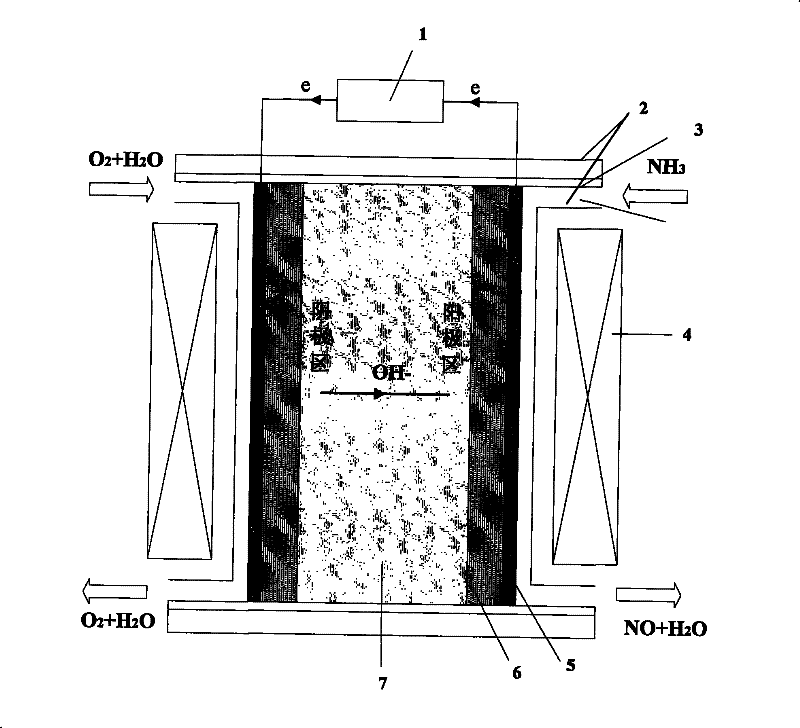
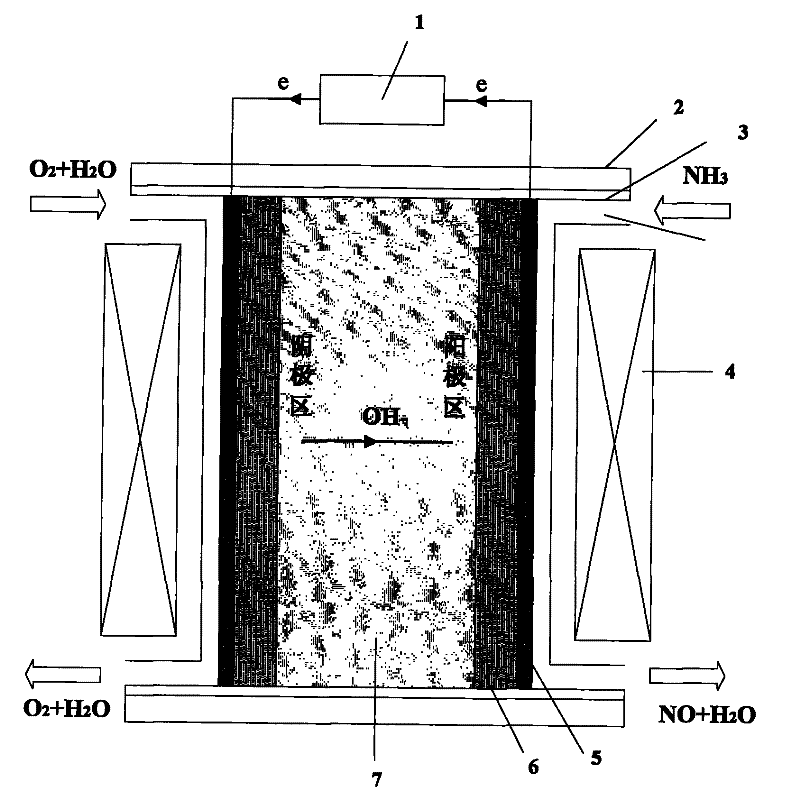
Manufacturing method of fuel cell apparatus using NH3 as fuel gas
A fuel cell and fuel gas technology, applied in fuel cells, battery electrodes, circuits, etc., can solve the problems of unspecified fuel cell preparation methods, anode carbon poisoning, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the anode load is 3mg / cm 2 The Pt / C membrane electrode and the quaternized naphthylbiphenyl polyethersulfone anion exchange membrane (BQAPPES) are hot-pressed to form a film (hot-pressing temperature is lower than 70°C, the pressure is about 1.0-2.0MPa), and the Pt net is attached The device is sealed and put into the single fuel cell mould. with pure N 2 Perform cathode and anode area purge, control the operating temperature range of the fuel cell at 80°C, and polarize. Will store 99.9% NH 3 The gas cylinder is opened, the mass flow meter controls the flow rate to 40ml / min, and the cathode is fed into O 2 , the intake flow rate is 40ml / min. Before closing the circuit, measure the open circuit voltage with an electronic multimeter to be 0.68V; close the circuit, adjust the load to 15Ω, and the maximum output current is 35mA. During the test, deionized water was continuously added dropwise to the upper end of the ion exchange membrane to keep the ion e...
Embodiment 2
[0035] Embodiment 2: the anode load is 3mg / cm 2 The Pt-Ru / C membrane electrode and the quaternized polyether sulfone ketone anion exchange membrane (BQAPPESK) are hot-pressed to form a membrane, and the Pt mesh current collector is attached, sealed and put into a single fuel cell mold. with pure N 2 Perform cathode and anode area purge, control the operating temperature range of the fuel cell at 80°C, and polarize. Will store 99.9% NH 3 The gas cylinder is opened, the mass flow meter controls the flow rate to 100ml / min, and the cathode is fed into O 2 , the intake flow rate is 70ml / min. Before closing the circuit, measure the open circuit voltage with an electronic multimeter to be 0.84V; close the circuit, adjust the load to 15Ω, and the maximum output current is 48mA. During the test, deionized water was continuously added dropwise on the upper end of the ion exchange membrane to keep the ion exchange membrane in a wet state.
Embodiment 3
[0036] Embodiment 3: the anode load is 3mg / cn 2 The Pt / C membrane electrode and the phenolphthalein-type polyethersulfone anion exchange membrane (SPEEK) are hot-pressed to form a film, and the Pt net current collector is attached, sealed and put into a single fuel cell mold. with pure N 2 Perform cathode and anode area purge, control the operating temperature range of the fuel cell at 20°C, and polarize. Will store 99.9% NH 3 The gas cylinder is opened, the mass flow meter controls the flow rate to 100ml / min, and the cathode is fed into O 2 , the intake flow rate is 70ml / min. Before closing the circuit, measure the open circuit voltage with an electronic multimeter to be 0.60V; close the circuit, adjust the load to 15Ω, and the maximum output current is 30mA. During the test, deionized water was continuously added dropwise on the upper end of the ion exchange membrane to keep the ion exchange membrane in a wet state.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com